Biomedical Engineering Reference
In-Depth Information
[53]. The cooling of the electrolytes results in the dissolution rate
of the TiO
2
that can be signiicantly lowered. They found that 1 h of
anodization results in formation of nanotubular self-organized TiO
2
structure with high degree of homogeneity, latness, and precipitates
free. Different anodization voltages (Fig. 9.49) clearly shows that at
1 V, there was only some pore formation observed, but considerable
dissolution of Ti takes place due to the fact that at this potential,
the Ti is in active dissolution region [53]. At potentials equal or
higher than 5 V, a self-organized nanotubular layer is formed. The
layer thickness and the tube diameter increases with increasing
applied potential [53].
(a)
(c)
(b)
(d)
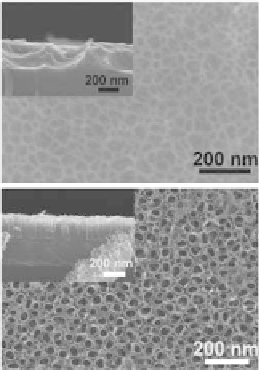
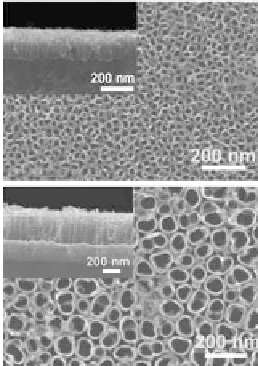
Figure 9.49
SEM images of sample anodized at 2
°C
in 1M H
2
SO
4
+ 0.15
wt% HF electrolyte at potentials: 1 V (a), 5 V (b), 10 V (c),
and 20 V (d). The insets show the side views of respective
structures [53].
The inluence of applied potential on the resulting diameter
and length of the nanotubes after 1 h of anodization is shown on
Fig. 9.50. At 5 V, the tubes have diameter of 30 nm and length of
180 nm, which increases at 20 V to 100 nm and 400 nm, respectively.
At higher potentials (25 V), no regular tubes were formed in
the Macak
et al
. setup [53]. Comparable results were achieved
for 1M H
3
PO
4
+ 0.3 wt% HF electrolytes [4]. The tube length and
diameter can be controlled in a wide range of applied potentials.
They found that for potentials between 1 and 25 V, tubes could
be grown with any desired diameter ranging from 15 to 120 nm