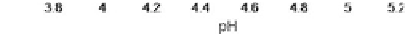Biomedical Engineering Reference
In-Depth Information
Figure 9.41
SEM cross-section of the TiO
2
nanotube coating and
precipitate layer formed by anodization of Ti for 2 h in 1M
H
2
SO
4
+ 0.1M NaF solution at a constant potential of 20 V
and pH 5.0 [13].
The pH and temperature plays an important role in the
nanotube length (Fig. 9.42, Fig. 9.43). The pH has no noticeable
effect on the diameter of the TiO
2
nanotubes, but the nanotube
length increases drastically with increasing electrolyte pH.
This effect is related to the enhanced chemical dissolution rate of
TiO
2
in acidic solutions. With increasing electrolyte temperature,
the rate of chemical dissolution increases, resulting in a thinner
ilm of the nanotubes.
Figure 9.42
Nanotube length (coating thickness) vs. pH for the anodization
of Ti for 10 h in 1M H
2
SO
4
+ 0.1M NaF solution at a constant
potential of 20 V, at 24
°C
[13].