Biomedical Engineering Reference
In-Depth Information
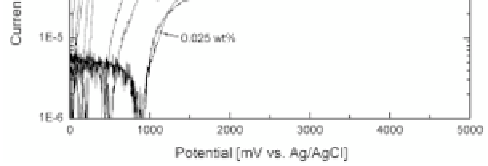
In the absence of the luorides, the surface is clearly spontaneously
passive. Addition of luorides leads to the shift of the open circuit
potential to the more negative values and the occurrence of an active
passive transition. For the concentrations of 0.2 and 0.3 wt%, a
second current increase occurs at about 1200 mV, which is related
with anodic pore formation [4].
Figure 9.14
Polarization curves recorded from 0 V to 5 V at a sweep rate of
5 mV/s for different concentrations of HF [4].
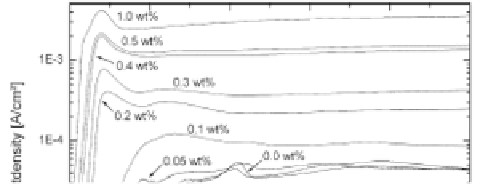
During anodization, it is possible to ind some characteristic
stages of anodic oxidation on the recorded current curve (Fig. 9.15)
[118]. Generally, the curve can be divided into four stages:
(i) In the irst stage, a compact oxide barrier layer is formed,
which leads the current to decrease signiicantly due to the
low conductivity of metal oxide.
(ii) In the second stage, some cracks and narrow slits appear on
the surface due to ield-enhanced dissolution of the oxide
layer, and the current starts to increase.
(iii) In the third stage, the current reaches a stable state, which
corresponds to the random formation of porous structure
in the slits and cracks. In this process, pore formation and
dissolution of the oxide layer is possible as well (equilibrium
of the pore formation with the pore dissolution).
(iv) In the fourth stage, when the dissolution rate is larger than
the pore formation rate, the porous structure is consumed
and current density decreases.