Biomedical Engineering Reference
In-Depth Information
alloy, but unfortunately these implanted alloys underwent pitting
corrosion [26]. This pitting corrosion is attributed to the reduced
oxygen content at the interface between the passive layer and the
implanted layer [45, 46]. The implanted ions act as a barrier to
the diffusion of oxygen. The change of the corrosion resistance of
the calcium-implanted Ti-6Al-4V alloy after long-term exposure
into SBF is related with formation of calcium phosphates. The Ca-P
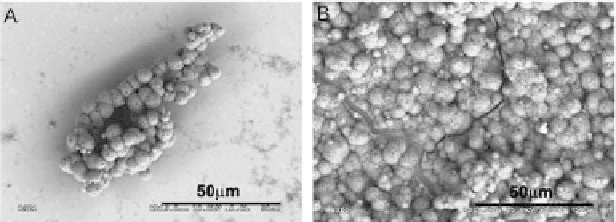
precipitations are more numerous after longer exposures (Fig. 5.32),
and the precipitated layer is continuous. The Ca/P ratio in the
precipitations on the implanted surface after the exposure for
720 h was about 1.66, which suggests hydroxyapatite formation.
Implantation of the calcium ions deinitely enhances the calcium-
phosphate precipitation.
Figure 5.32
Surface of the Ti-6Al-4V alloy implanted with a 1 × 10
17
Ca
+
/
cm
2
after exposure in SBF: (A) 168 h and (B) 720 h [26].
5.4
Corrosion of the Other Dental Materials
In medicine, the stainless steels which are typically used contain
17-20% Cr, 13-15% Ni, 2-3% Mo, and small amounts of other
elements. Cr is the element responsible for the high passivation
ability of these alloys. An increase in Cr and Mo content leads to
an increase in the resistance against localized corrosion. Although
the main problem is related with Ni, it is commonly added as
the austenite forming element, but unfortunately, highly toxic
for the human. The other Co-Cr-type alloys have high corrosion
resistance [53]. The Co-Cr alloys are superior to stainless steel, both
in fatigue and wear resistance. In all Cr-containing alloys, Cr
2
O
3
forms on the surface acting as a corrosion protective layer.
Taher and Al Jabab [50] investigated the galvanic corrosion
behavior of a different commercial dental alloys (Table 5.11) coupled