Biomedical Engineering Reference
In-Depth Information
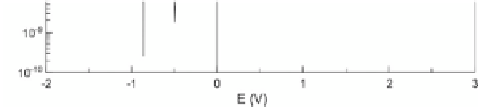
Ti, which is extremely important in biomaterials applications. The
porous TiO
x
has wide passive range (plateau on the polarization
curve). This passive range suggests that the surface oxides are
stable and no pitting occurs. The current density value in the
passive range is almost the same in both cases. For pure Ti at higher
positive potentials, a transpassivation and transformation of oxide
into soluble salt is observed, which results in fast metal dissolution
(a). This behavior was not observed for the porous TiO
x
(b) and the
surface layer protect material against dissolution.
Figure 5.22
Potentiodynamic corrosion curves for pure Ti (a) and porous
TiO
x
layer (b) in Ringer's electrolyte [14].
The Ti-glass or Ti-HA bionanocomposites are a new class of
biomaterials, with interesting mechanical and biomedical properties,
prepared by Niespodziana
et al
. [33, 34]. The corrosion properties of
the Ti-glass (45S5-Bioglass) were potentiodynamically investigated
in Ringer's solution by Jakubowicz
et al
. [16] (Fig. 5.23). After
electrochemical etching, the surface of Ti-glass nanocomposites has
a slightly better corrosion resistance (b) than the parent not etched
sample (a). The corrosion current density, estimated from the Tafel
extrapolation, of the nanocomposite before and after electrochemical
treatment is 5.1 × 10
-6
A/cm
2
and 1.87 × 10
-6
A/cm
2
, respectively.
The lower corrosion current density indicates better corrosion
resistance and lower corrosion rate. The surface after etching is
covered by thicker oxide, than samples not etched with native oxide.
These anodic oxides provide better corrosion resistance of the
electrochemically treated Ti-45S5 bionanocomposites [16].