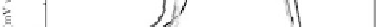Biomedical Engineering Reference
In-Depth Information
measured corrosion properties of Ti alloys consisting 0.1-2 wt% Pt
and Pd in artiicial saliva containing 0.2% NaF (corresponding to 905
ppm F). The addition of over 0.5% Pt or Pd to Ti results in formation
of a passive ilm on the Ti surface and hence high corrosion resistance
in the Ti-Pt or Ti-Pd alloys [20].
During anodization of the Ti or its alloys, nanotubes (see
Chapter 9) are often formed. The nanotubes formed in the
anodization of Ti-based materials are a possible material for
dental application and are investigated with respect to corrosion
resistance too. For example, anodization of the Ti-Nb alloys results
in the formation of oxide nanotubes with diameters range from
55 nm to 220 nm and lengths range from 730 nm to 2 μm [20]. These
nanotubes show interesting corrosion properties (Fig. 5.18) [37].
The corrosion current density, for the non-anodized alloy (Fig. 5.18a)
decreases with increasing Nb content. The lowest
I
corr
occur for the
Ti-40Nb alloy. The increase of corrosion resistance with Nb content
is attributed to rapid formation of a passive mixed TiO
2
and Nb
2
O
5
ilm of a few nanometers thick on the Ti-Nb alloy surface. Anodic
current rises in a transpassive region, indicates pitting corrosion,
due to a breakdown of the TiO
2
and Nb
2
O
5
ilm. Ti-xNb alloys
with the oxide nanotubes on the surface (Fig. 5.18b) show lower
corrosion resistance, than for the non-anodized alloys. It is seen that
E
corr
is lower and
I
corr
is higher for the Ti-Nb alloys with the surface
nanotubes. The passive range is wider for the alloys with nanotubes,
which means that the mixed TiO
2
and Nb
2
O
5
ilm is stable.
Figure 5.18
Potentiodynamic polarization curves of Ti-xNb alloys
immersed in aqueous 0.9% NaCl solution at 36.5 ± 1°C:
(a) non-anodized alloy and (b) anodized alloy [20]. See also
Color Insert.