Biomedical Engineering Reference
In-Depth Information
Lopez
et al
. [29] investigated corrosion behavior (in Hank's
solution consisting 8 g/L NaCl, 1 g/L glucose, 0.4 g/L KCl, 0.35
g/L NaHCO
3
, 0.14 g/L CaCl
2
, 0.1 g/L MgCl
2
· 6H
2
O, 0.06 g/L
Na
2
HPO
4
· 2H
2
O, 0.06 g/L KH
2
PO
4
and 0.06 g/L MgSO
4
· 7H
2
O) of
three V-free Ti alloys: Ti-7Nb-6Al, Ti-13Nb-13Zr, and Ti-15Zr-
4Nb. The presence of Nb in all alloys stabilizes the β phase. The
Ti-13Nb-13Zr sample is richer in β phase than Ti-7Nb-6Al and
Ti-15Zr-4Nb alloys. The Ti-13Nb-13Zr and Ti-15Zr-4Nb have very
low passivation current densities, lower than those of the Ti-7Nb-
6Al as well as Ti-6Al-4V, and these materials exhibit an excellent
resistance to pitting corrosion [29]. In the Ti alloys, Al and Zr form
stable protective passive layers. The higher concentration of Zr in
the passive layer of the Zr-containing alloys, measured by Lopez
et al
. [29], as compared with the Al concentration in the passive layer
of Ti-7Nb-6Al, explain the lower current densities observed in the
irst case.
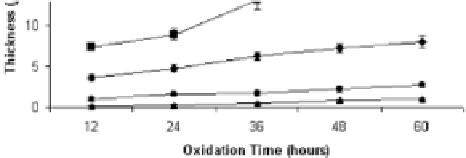
The thermal oxidation of the Ti-6Al-4V alloy was found to
produce corrosion and wear resistant surface layers [9]. Oxide
thickness and oxygen diffusion zone depth steadily increases with
increasing oxidation temperature and time (Fig. 5.9). Oxide layers
are composed from anatase and rutile. Additional signiicant increase
in the surface hardness (from 3500 to 9000 HV) was achieved
due to formation of a hard oxide layer and an oxygen diffusion
zone [26]. Oxidation at 600°C for 60 h produced the most corrosion
and corrosion-wear resistant surface (in 5M HCl, 0.9% NaCl,
respectively) of the Ti-6Al-4V alloy.
Figure 5.9
The effect of oxidation time and temperature on thickness of
OL (oxide layer) and ODZ (oxygen diffusion zone) [26].