Biomedical Engineering Reference
In-Depth Information
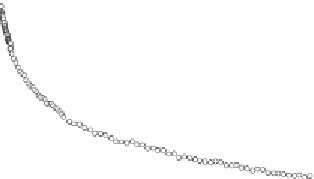
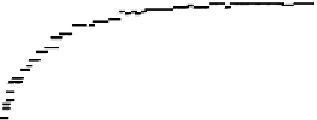
Figure 11.
Contact angle and drop base of a water drop, containing a non-ionic surfactant on a hy-
drophobic surface: (
!
) contact angle; (
1
) drop diameter.
A general form of the spreading rate law in such case is given by Eyring's bi-
exponential form [25]
d
r
d
t
=
A
A
e
−
b
A
cos
θ
A
R
e
b
R
cos
θ
,
−
(3)
where
θ
is dynamic contact angle, i.e., the contact angle as a function of time
θ
θ(t)
. The indices A and R describe the parameters of advancing and reced-
ing movement of the TPC line. For
θ>
, the TPC-line is advancing, e.g., in the
case of spreading of aqueous surfactant solutions; for
θ<
, the TPC-line is reced-
ing, e.g. during evaporation of a water drop. The spreading law (3) reflects the
barrier character of the TPC line movement [26], which was confirmed for the
adsorption-desorption mechanism of the TPC movement [37, 38]. Most of wet-
ting characteristics such as mobility and immobility of the TPC line—pinning ef-
fect [39], quasi-static advancing and receding contact angles, characteristic spread-
ing velocity—can be interpreted with the help of equation (3). The parameters
A
A
,
R
and
b
A
,
R
depend on a particular mechanism, but in the case of equilibrium
d
r/
d
t
=
=
0, the following relationship is valid
1
b
A
+
b
R
ln
A
A
=
cos
A
R
.
(4)
Thecosineof
is defined by the Young equation (1) and does not depend on any
mechanisms. From a simple geometrical consideration [40, 41], the base radius
r
for a small drop can be expressed as
cos
θ)
3
/
2
3
π
(
1
+
r
3
=
cos
θ)
V,
(5)
(
1
−
cos
θ)
1
/
2
(
2
+
where
V
is the drop volume, which is supposed to remain constant, and
θ
is the
contact angle changing with time. In Fig. 11, the contact angle and base radius of
an evaporating drop (water) are illustrated.

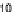
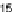
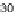

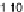
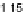

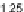

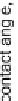

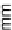







































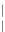






























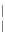



































































































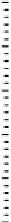














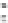




















































































































































































































































































































































Search WWH ::

Custom Search