Biomedical Engineering Reference
In-Depth Information
Figure 17.
Molecular structures of the alkyl polyethoxylates (left) and trisiloxane surfactants (right).
Grey atoms are oxygen; black atoms are carbon; small white atoms are hydrogen; and large white
atoms are silicon [105].
2 mmol/m
−
3
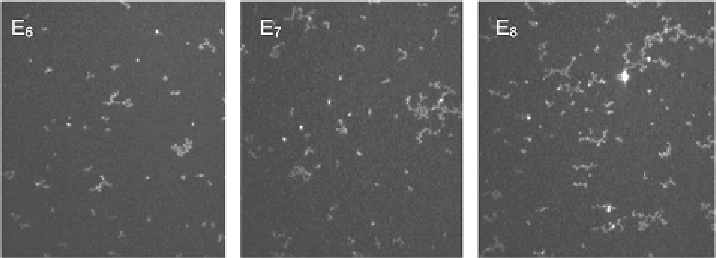
Figure 18.
BAM images for trisiloxanes E
6
,E
7
,E
8
at
C
=
[121].
studies which confirmed slightly higher contact angles and surface tension values
for E
4
and E
5
[110, 121]. Optimal wetting abilities were found for chain lengths
in range of 6-9 ethoxy groups. Silwet
®
L-77 in particular, and a number of simi-
lar commercial products, with an average of
n
=
7
.
5 were found to have the most
advanced wetting properties [105].
Recently, Ritacco
et al.
[121, 122] have measured dynamic surface tensions for
trisiloxane surfactants for the short time (
<
1 s). They discovered that in the case of
trisiloxanes (with relatively long EO
n
chains,
n
6-9) possessing superspreading
character, two inflection points were detectable on dynamic surface tension curves
[121]. Using Brewster Angle Microscopy, the authors directly observed aggregates
appearing on the solution interface for those trisiloxanes in a certain range of con-
centrations, Fig. 18. This allows us to suggest that the surfactant molecules are
present at the liquid/air interface in two states: as monomers and as surface aggre-
gates [121, 122]. The aggregates could act as reservoirs of surfactant monomers
in the course of spreading, completely confirming the suggestion made by Kumar
=

Search WWH ::

Custom Search