Biomedical Engineering Reference
In-Depth Information
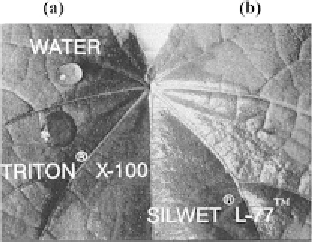
Figure 15.
Photograph [77] depicting the spreading of: (a) a water drop and a drop of 0.25% Triton
®
X-100 solution, (b) 0.1% Silwet
®
L-77 solution on a velvetleaf surface.
−
C(O)CH
3
. Until recently, a trisiloxane molecule with eight
poly(oxyethylene) groups was generally referred to as E
8
.
However, a large selection of commercial trisiloxane products is available on the
world market nowadays and these are called correspondingly by their registered
trade names. Trisiloxanes possess an unusual ability to induce highly efficient wet-
ting properties even on extremely hydrophobic surfaces. It has been reported that
the addition of trisiloxane surfactants can transform a small spherical drop into an
'infinitely' thin liquid film—the phenomenon popularly known as 'superspreading'
[99]. Thus, trisiloxane surfactants are widely recognized as 'the superspreaders'.
Due to its unique characteristics and colossal practical employment, the super-
spreading phenomenon has attracted much attention, especially from the theoretical
point of view. In spite of considerable research interest, there is still a lack of
explanation of the underlying mechanism of superspreaders' behaviour and under-
standing of the necessary conditions for its realisation. Superiority of trisiloxanes
over conventional surfactant types has been confirmed in numerous publications
over the years [67, 77, 99-107].
Silwet
®
L-77
™
, a commercially available trisiloxane surfactant (polydispersed
with an average of 7.5 ethoxylate groups), has been widely used since the late eight-
ies. Aqueous drops with sufficiently high bulk concentration of trisiloxanes spread
rapidly, even when placed on hydrophobic surfaces, and completely wet the solid
with no measurable final contact angle at the contact line [99, 108].
It is believed that the overall wetted area achievable by an aqueous droplet con-
taining trisiloxane surfactant can be as much as 50 times greater than pure water,
and 25 times more effective than a conventional surfactant [77]. Figure 15 illus-
trates this point by comparing the relative spreading properties of water with 0.25%
Triton
®
X-100 (polyoxyethylene (10)-octylphenyl ether), and with aqueous solu-
tion of 0.1% Silwet
®
L-77 surfactant on a velvetleaf (
Abutilion theophrasti
). Water
alone on velvetleaf makes a contact angle bigger than 90
◦
; aqueous Triton
®
X-100
solution slightly reduces the contact angle due to a decrease in surface tension and
gives a bigger spreading area. However, Silwet
®
CH
3
,or
−
Ac; Ac
=−
L-77 provides a most significant
Search WWH ::

Custom Search