Biomedical Engineering Reference
In-Depth Information
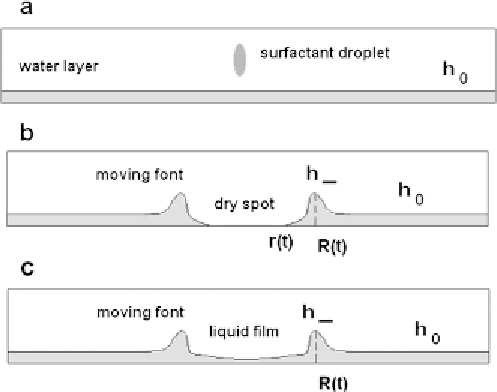
Figure 8.
(a) A small droplet of aqueous surfactant solution is deposited on a top of thin aqueous
layer of thickness
h
0
; (b) dry spot formation in the centre: cross section of the system:
R(t)
—radius
of a circular moving front,
r(t)
—radius of dry spot in the centre,
H
—the height of the moving front;
(c) the same as in the previous case (b) without dry spot formation in the centre.
reach
0.75. It was also shown in [46], that formation of the dry spot in the centre
is determined by the speed of the first stage: the higher this speed, the higher the
probability to have dry spot formation. Hence, the dry spot forms in the case of
soluble surfactants and does not form in the case of insoluble surfactants.
The observations in [46] differed from the earlier theoretical model [48], thus the
influence of surfactant solubility and disintegration of micelles was incorporated to
improve on the previous theoretical model. According to theoretical predictions
[46], low soluble surfactant produce faster first and second stages. Low soluble sur-
factant (Tween
®
20) produced a power law exponent 0
.
73
∼
0
.
01, being close to the
maximum attainable spreading rate 0.75 predicted theoretically. For the highly sol-
uble surfactant DTAB, the solubility was most significant during the second stage
where the spreading front reached some final position and did not move any further
in the agreement with theoretical predictions.
Chan and Borhan [49] deduced that insoluble monolayers enhance the over-
all spreading rate. They developed a mathematical model for surfactant enhanced
spreading that suggests two additional mechanisms which influence the spreading
rate: the development of positive surface curvature near the moving contact line,
which produces a favourable radial pressure gradient within the drop, and the sur-
factant convection in a vicinity of the moving contact line. Accumulation of the
surfactant at the contact line, due to surface convection, leads to faster spreading
of a drop. Seguin
et al.
[50] performed experiments using ionic and non-ionic sur-
factants in different solvents. For ionic surfactants, effect of a charged head group
on micellisation was obvious, while for non-ionic pure ethylene glycol appeared to
±
Search WWH ::

Custom Search