Biomedical Engineering Reference
In-Depth Information
(a) (b)
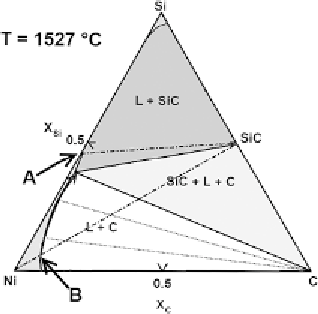
Figure 16.
Ternary phase diagrams computed at 1170
◦
C and 1527
◦
C (adapted from [206]).
[205] and Ag-5Si/SiC [195] systems that, when the original content of Si in Ni is
above
40%, the equilibrium is established between the Ni-Si alloy and SiC. After
a small dissolution of SiC the saturated liquid alloy does not react anymore with the
solid substrate, leaving an undisturbed interface.
In close similarity with what already discussed in the case of diborides systems,
this behaviour can be interpreted by means of the ternary phase diagrams X-Si-C.
In the specific case of Ni-Si-C diagram [206], it is seen (Fig. 16) that the tie-
line between the pure Ni and the SiC compound crosses first of all, in point B the
liquidus line: here, the liquid phase has already dissolved a small quantity of SiC.
From this point onwards, the composition of the liquid follows the arrow becoming
enriched in Si and segregating C until a three-phase region is reached with the
simultaneous presence of SiC
∼
Liquid. It is then clear that when pure Ni is
put in contact with SiC both dissolution of the substrate and reprecipitation of C
takes place. However, the same diagram shows that additions of Si from
X
Si
≈
+
C
+
0
.
4
upwards can limit or even suppress the substrate dissolution leaving only two phases
at equilibrium, i.e., the nearly pure Ni-Si alloy and the solid SiC. It is thus clear that
ternary Me-Si-C phase diagrams should be established for a number of systems as
large as possible in order to allow a wider access to their isothermal sections related
to the experimental temperatures.
These results have been recently confirmed in our Laboratory, in a study of the
wetting of SiC by Si-rich Ni alloys [204]. The spreading kinetics of Ni-56Si on
SiC at three temperatures (1100
◦
C, 1200
◦
C and 1350
◦
C) is shown in Fig. 17. The
spreading rate in the first, fast stage obviously increases with temperatures increas-
ing (Fig. 17a). According to the changes of drop dimensions (base diameter and
drop height) and contact angle, the spreading does not reach the contact equilibrium
completely at 1200
◦
C after a holding time of 1 hour, and the contact angle curve can
be separated into three parts, I, II and III (Fig. 17b). The spreading rates in the three

Search WWH ::

Custom Search