Biomedical Engineering Reference
In-Depth Information
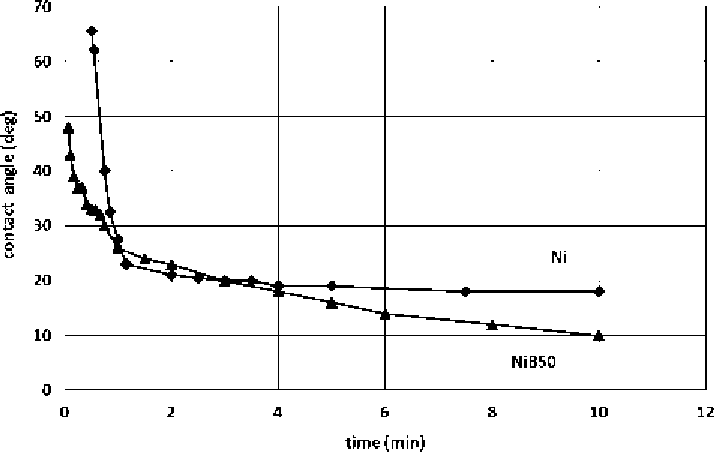
Figure 11.
Kinetics of wetting of Ni (
)andNiB50(
Q
)onHfB
2
at 1520
◦
C.
Wetting Results
. On the basis of these thermodynamic considerations, sessile
drop experiments have been made, at 1520
◦
C, using different Ni-B alloys compo-
sition. In particular, the results regarding pure Ni and the Ni-50 at%B intermetallic
compound (NiB50) which undergoes a peritectic reaction at 1035
◦
C [93] are briefly
discussed here.
The behaviour of contact angles
vs.
time for the Ni alloys in contact with the
HfB
2
substrate at 1520
◦
C is shown in Fig. 11.
Two results must be underlined: (1) the largest part of the solid-liquid interac-
tions occur during the first minute of contact, where, in the case of pure Ni, strong
dissolution of the substrate takes place, and, in the case of the NiB50 alloy, where
no dissolution at all has been found; (2) in the case of dissolution, the contact an-
gles remain nearly constant after about two minutes of contact, while in the case of
wetting without dissolution the contact angle keeps decreasing for a long time and
seems not having reached an equilibrium value even after 10 minutes, with a final
contact angle
<
10
◦
.
As shown in Fig. 10b, the isothermal section at
T
1527
◦
C shows that the
=
NiB50-HfB
2
line crosses the liquidus line at
X
Hf
=
0
.
03. This means that a very
little amount of HfB
2
dissolves into the liquid phase, which however reduces to
nearly zero already at 1130
◦
C as shown by other diagrams not reported here [58].
The drop-ceramic cross sections are presented in Fig. 12: a striking difference
is seen in the interface structure for the two cases. With pure Ni, the formation of
a sigmoidal interface profile, is the result of the competition between the rapid dis-
solution of HfB
2
in the liquid phase, the pinning of the liquid drop at the triple line
Search WWH ::

Custom Search