Biomedical Engineering Reference
In-Depth Information
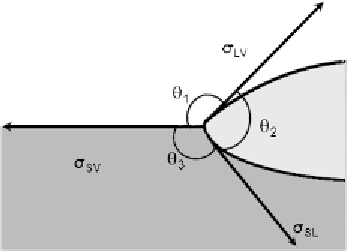
Figure 2.
Equilibrium configuration in the presence of dissolution of the solid.
2. Dissolutive Wetting
As already mentioned, at high temperature, the atom mobility is also high so that at
least some dissolution of the solid phase into the molten matrix can take place until
the chemical potential of the diffusing species is the same in the solid and liquid
phases [37, 43, 45-51]. Even if no reactions occur, the composition of the liquid
phase changes with time; as a consequence, also its surface tension changes and a
dynamic condition is set up, where both contact angle and drop dimensions (base
diameter, height and also volume) are a function of time. The solid-liquid interface
does not lie anymore on a plane, but a groove under the drop forms with the 'clas-
sical' shape of a (nearly) spherical cup (Fig. 2). With the immediate consequence
that the Young law (Eq. (1)) is no longer valid, as the triple line equilibrium must
take into consideration also the vertical components of the surface tension vectors.
Indeed, at the new triple line, the interfacial tension vectors reach an equilibrium
configuration expressed by the so-called Neumann rule (Fig. 2):
σ
SL
sin
θ
1
=
σ
SV
sin
θ
2
=
σ
LV
sin
θ
3
.
(7)
Thus, in this case, what is measured in a sessile drop test is the 'apparent' contact
angle, that is the one 'above' the plane defined by the solid surface (
in
Fig. 2). This dissolutive stage of the wetting process is usually very fast; indeed, as
noted for ex. in [45], dissolution is often increased by solutocapillary Marangoni
movements which arise near the triple line, due to differences in surface tension
between the drop apex (richer in atoms of the metal matrix) and the triple line,
where the concentration of atoms dissolved out of the solid support is high. These
processes give rise to 'Grooves' and/or 'Ridges' of the micrometer size (Figs 3
and 4). They have been shown to occur, at the microscopic scale, in many systems at
high temperature, even with high melting point ceramic materials and with systems
which, at the macroscopic scale, are considered non-reactive such as Ni/sapphire
[44, 52-57].
As the dissolution process changes the liquid-vapour and the solid-liquid sur-
face tensions, a new contact angle should result. If the new angle is lower than the
[π −
θ
1
]

Search WWH ::

Custom Search