Biomedical Engineering Reference
In-Depth Information
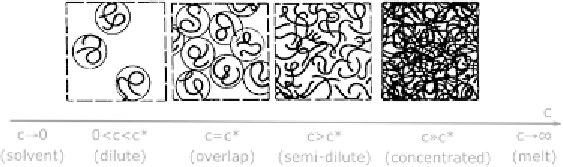
Figure 20.
Solution regimes of flexible polymers in a Newtonian solvent.
Unfortunately, this technique is only applicable to relatively viscous liquids, as the
filament breaks up too rapidly for low-viscosity samples. The alternative stagnation
point devices, such as the opposed nozzle rheometer [80], do offer a stationary flow,
but the residence time of a polymer chain in the elongational flow field is typically
quite short, has large statistical fluctuations and depends on the rate of elongation.
Therefore, a steady-state value for the elongational viscosity is very hard to obtain.
At a molecular level, the energy dissipation mechanism for elongational flows
can be explained in terms of the interaction between the additive and the surround-
ing fluid, which is essentially due to hydrogen bonds between water molecules and
monomers. Thus, when the polymer is coiled, the only monomers affected by the
interaction are those located in the external shell, and the polymer molecule behaves
like a spherical particle advected by the flow. As the velocity gradient increases the
polymer stretches [81], and therefore more of its monomers become affected by the
interaction with the fluid, increasing molecular friction and hence viscous dissipa-
tion.
Common viscoelastic fluids are solutions of long-chain polymers in a Newtonian
solvent (e.g., water). The macroscopic physical properties of these solutions are
strongly dependent on the polymer concentration, as illustrated in Fig. 20. For low
concentrations, the average distance among polymer molecules is larger than their
size, so that they do not interact with one another: polymers exhibit a random coil
conformation and can be described as spherical particles suspended in the fluid
(dilute regime). As the polymer concentration grows, the average distance between
polymer molecules reduces until the monomers placed on the external shell of one
coil can interact with monomers of other coils. This happens for a critical value
of concentration (the overlap concentration), and marks the beginning of the semi-
dilute regime, where polymer chains are randomly entangled and can be no longer
described as coils. Dilute solutions are of particular importance in many engineering
applications, because due to the low polymer concentration their viscosity is very
similar to that of the pure solvent, although the solution exhibits viscoelasticity.
E. References
1. Reynolds, O., On the action of the rain to calm the sea. Papers on Mechanical and Physical Sub-
jects, vol. 1, pp. 86-88. Cambridge University Press, 1900.
Search WWH ::

Custom Search