Biomedical Engineering Reference
In-Depth Information
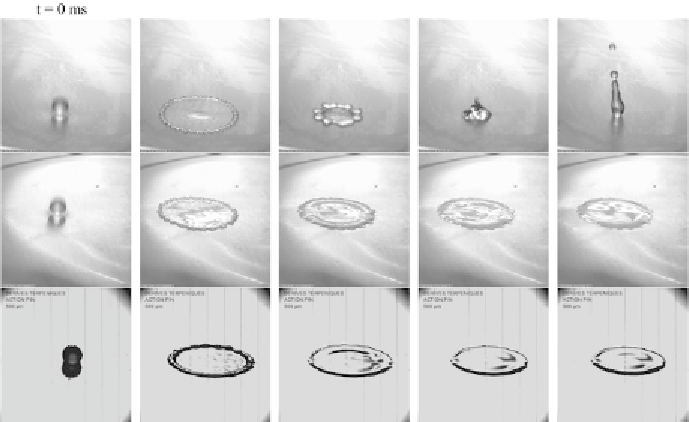
Figure 8.
Impact of a liquid droplet (
D
0
=
150) on a hydrophobic Parafilm surface:
a drop of pure water (a) bounces off the surface, while both a dilute solution of polyethylene oxide (b)
and a solution of a high-mobility surfactant can prevent drop rebound.
2
.
5 mm, We
=
D. Impact of Viscoelastic Drops
D.1. Impact on Homo-Thermal, Hydrophobic Surfaces
The study of viscoelastic drops received considerable attention after the accidental
discovery that small amounts (of the order of 100 ppm) of Polyethylene Oxide
(PEO) can reduce the tendency of drops to rebound after impacting on hydrophobic
surfaces, which can be exploited to control many spray applications [8, 29, 30].
A qualitatively similar result can be achieved, as shown in Fig. 8, by using cer-
tain surfactants hence changing the dynamic wetting properties of the liquid [31,
32]; however, the surface tension and shear viscosity of aqueous solutions of these
flexible polymers in the dilute regime are known to be almost identical to those of
the pure solvent. A similar independent study demonstrated that polymer additives
also increase the critical Weber number for drop splashing [33]; moreover, drop im-
pact control can be achieved by means self-assembling surfactant systems creating
rod- or worm-like micelles [34].
Having ruled out the influence of capillarity and viscosity, it was suggested [31]
that these effects are related to the elongational viscosity of the fluid, defined as the
ratio of the first normal stress difference to the rate of elongation of the fluid, which
for a polymer solution can be two or three orders of magnitude higher than that of
water [35]. In particular, it was argued that the rapid deformation of drops upon
impact can induce a coil-stretch transition [36] of the molecules in the bulk fluid,
resulting into a transient increase of the elongational viscosity; the large energy
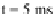




































Search WWH ::

Custom Search