Biomedical Engineering Reference
In-Depth Information
Figure 8.
Highly pure water surface tension variation.
Figure 9.
Water evaporation in air.
Figure 10.
Water evaporation over a Teflon substrate in a fluent Argon environment.
hydrocarbons were dispersed. It can be appreciated that the simple effect of CO
2
derived by human breathing is quite able to reduce in the first exposure minutes the
original surface tension value.
A simple example of a macroscopic application aiming to show the combined ef-
fects of atmosphere contaminants and evaporation kinetic is reported in Figs 9 and
10. Figure 9 shows the temporary evolution of a high purity macroscopic water
drop placed upon a flat Teflon substrate (static contact angle
109-112 (deg)
[184]) by evaluating two superimposed photos of the same drop collected during
a 210 minutes long evaporation path in normal air. Profile 1 (back) is the
t
=
0drop
while profile 2 (front) represents how the drop was looking after 210 minutes. The
photographic set remained untouched and pictures were obtained by using remote
controls. During this time the drop reduced its volume but also the overall shape
changed, giving the false impression that the
effective
contact angle value moved
down from an original true hydrophobic condition to an apparent more hydrophilic
one.
=







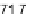
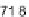
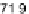
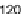




























Search WWH ::

Custom Search