Biomedical Engineering Reference
In-Depth Information
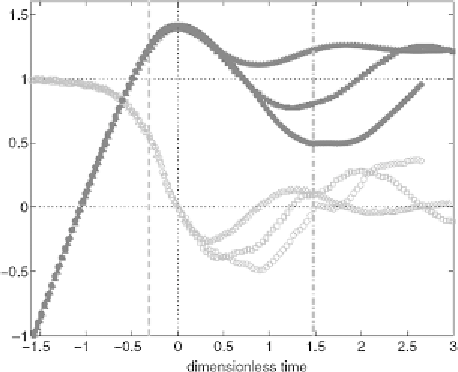
Figure 6.
Normalised position of bubble centroid (filled symbols), 2
z/d
eq
+
2, and normalised ve-
locity (open symbols),
(
d
z/
d
t)/U
,
versus
the normalised time,
tU/d
eq
, for different modified Stokes
numbers,
St
∗
=
3
.
9 (triangles), 10.8 (squares) and 21.1 (circles) [82]. The zero time is off-set to the
zero bubble velocity. The dash lines describe the bubble positions away from the solid surface by one
equivalent diameter.
when normalised to account for deformation (Fig. 6). This aspect is an important
consideration for modelling the drainage process.
The surface wettability and microscopic roughness have been found to signif-
icantly influence the bubble rebound and oscillation [37, 81]. In particular, there
is a significant difference between the bubble oscillation at the hydrophobic and
hydrophilic surfaces at long time. At the hydrophobic surface (such as Teflon),
the intervening liquid film was ruptured and the three phase contact was formed.
However, at the hydrophilic surface (such as mica) the bubble arrested beneath the
surface and no direct contact was formed. The interchange of the bubble kinetic
to surface energy during the collision with the hydrophobic Teflon surface is also
lower than that with the hydrophilic (mica and glass) surfaces. The difference is
due to the presence of sub-microscopic air pockets present on the rough hydropho-
bic Teflon surfaces which cause an earlier film rupture during the bubble rebound
and oscillation. Surfactants such as simple alcohols effectively reduce not only the
bubble rise velocity [35, 46] and hence the kinetic energy of the bubble rise, but
also significantly change the bubble rebound and oscillation at the hydrophobic and
hydrophilic surfaces [36, 37]. Specifically, surfactant mixtures can synergistically
adsorb at both the bubble surface and the solid surface, and change the surface prop-
erties, such as the surface charge and hydrophobicity, of both the bubble and solid
surfaces [39]. These surface properties ultimately change the surface forces and the
liquid film drainage and rupture, as described in Sections D and E.
Search WWH ::

Custom Search