Biomedical Engineering Reference
In-Depth Information
1. Mass Transport Inside the Drop: the Coffee Stain Effect
It is common experience that when a spilled drop of coffee dries on a solid surface, it
leaves a dense, ring-like deposit along the perimeter, or TPCL. The coffee, initially
dispersed homogeneously in the entire drop, becomes concentrated into a small
fraction of it. Such ring deposits are found wherever drops containing dispersed
solids or polymers evaporate on a surface. They affect processes such as printing,
washing and coating. By now the mechanism responsible for the coffee-stains on
surfaces has been widely understood [48, 57-69]. The characteristic pattern of the
deposition are due to a form of capillary flow in which pinning of the TPCL of the
evaporating drop ensures that liquid evaporating from the rim is replenished by liq-
uid from the interior. The resulting outward flow can carry virtually all the dispersed
material to the rim. This mechanism, first described quantitatively by Deegan
et al.
[57], predicts a distinctive power-law growth of the ring mass with time, a law inde-
pendent of the particular substrate, liquid or deposited solids. The model has since
then been substantiated by various experimental works and further theoretical stud-
ies. The qualitative observations show that rings are formed for a wide variety of
substrates, dispersed materials (solutes), and carrier liquids (solvents), as long as
(i) the solvent drops forms a non-zero contact angle with the surface; (ii) the TPCL
is pinned to its initial position for almost the entire evaporation time; and (iii) the
solvent evaporates, i.e., its vapour pressure is higher than zero. Deegan
et al.
[57]
also could rule-out other mechanisms which typically are responsible for solute
transport in liquids, like surface tension gradients, solute diffusion, electrostatics
and gravity, and found that they can be neglected in the ring formation process.
The phenomenon is basically due to a geometrical constraint: the free surface, con-
strained by the pinned TPCL, pushes the fluid outwards to compensate for the losses
due to evaporation, taking place all over the free surface of the droplet.
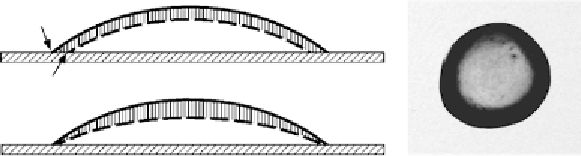
Figure 10 illustrates the factors leading to the outward flow in a small, thin,
dilute, circular drop. The evaporative flux continuously reduces the height of the
Figure 10.
Schematic illustration of the origin of the advective current. (A) When the TPCL is not
pinned, uniform evaporation removes the hashed layer, the interface moves from the solid line to the
dashed line, and the TPCL moves from position 1 to 2. (B) If the TPCL is pinned the motion from
1 to 2 must be prevented by an outflow to replenish the liquid removed from the edge (arrows). The
interface of the drop thus moves differently in order to keep the TPCL fixed. (C) Dried coffee ring
on a table. [A and B: Reprinted figure from: Deegan, R. D.; Bakajin, O.; Dupont, T. F.; Huber, G.;
Nagel, S. R.; Witten, T. A.,
Physical Review E
2000
, 62, (1), 756. Copyright (2000) by the American
Physical Society. Reproduced with permission. C: a coffee stain on the authors' desk.]









Search WWH ::

Custom Search