Biomedical Engineering Reference
In-Depth Information
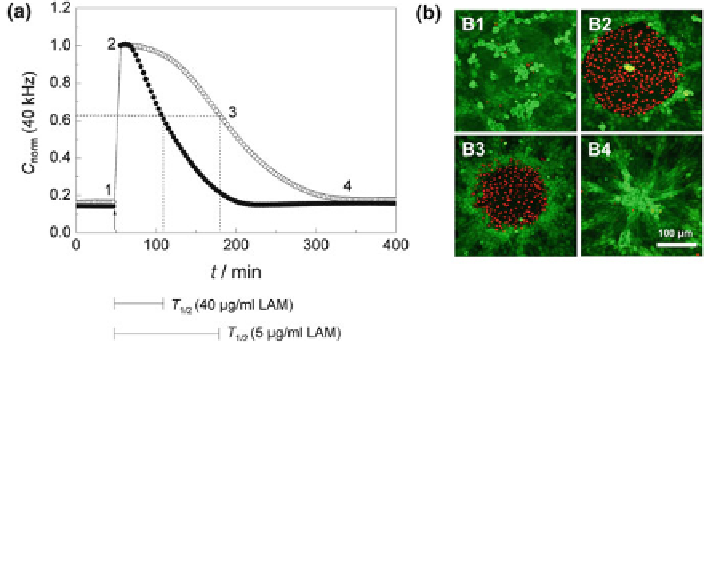
Fig. 12 ECIS-based cell migration assay. a Time course of the normalized capacitance measured
at an AC frequency of 40 kHz along a complete wound healing/migration assay with NRK cells
grown on ECIS electrodes pre-coated with 40 lg/mL (filled circle)or5lg/mL (open circle)
laminin. Numbers 1-4 indicate the time points at which a vital stain of the cells on the electrode
was performed (cf. Fig.
12
b). T
1/2
is the time to reach half-maximal repopulation of the electrode.
Capacitance data was normalized to the first value after electrical wounding (cell-free electrode).
b Fluorescence micrographs of NRK cells stained with calcein AM and ethidium homodimer-1 at
different points of the wound healing/migration assay (green = vital cells, red = dead cells; the
staining was performed 1: before, 2: immediately after the wounding pulse, 3: after 50% wound
healing, and 4: after complete wound healing)
readings [
50
]. In contrast to the mechanical scratch assay, the ECIS-based wound
healing assay provides well defined and highly reproducible wounds, as they
correspond to the size of the electrode.
Similar to cell attachment and spreading studies, the capacitance at a sampling
frequency of 40 kHz is the most useful indicator for monitoring the repopulation of
the electrode surface by cells migrating in from the periphery. Figure
12
a shows
the time course of the normalized capacitance at 40 kHz after wounding a
confluent layer of NRK cells that have been grown on ECIS electrodes pre-coated
with laminin (LAM) in different concentrations. The arrow at position 1 marks the
time point when the invasive electric field was applied to the cells, killing the cells
on the electrode. Immediately after pulse application the capacitance increases
from its minimum value, which mirrors a confluent cell layer, to a typical reading
for an open, cell-free electrode. The electrical permeabilization of the cell mem-
branes allows the current to flow freely through the dead cells without a mea-
surable capacitance contribution from the cell membranes. As time progresses
C
norm
continuously decreases back to the pre-pulse values as the electrode is
gradually repopulated by cells that migrate in from the periphery. The rate of the
capacitance decrease depends on the LAM concentration. Eventually, the capac-
itance reaches the stationary level of a cell-covered electrode again, indicating that
the healing process is completed (closure of the wound). To characterize the assay
readout for different electrode coatings by a single parameter, it is useful to
Search WWH ::

Custom Search