Biomedical Engineering Reference
In-Depth Information
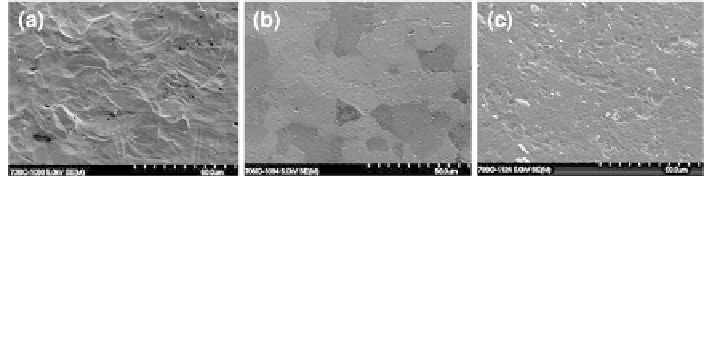
Fig. 2 Scanning electron micrographs of titanium and its polished counterparts. a Standard
commercially pure titanium as used in clinics (R
a
& 1 lm). b Electropolished titanium—
implants are submerged in an electrolyte solution and a charge is applied. Material is removed
from the surface at a rate that is dependent on the electrical conductivity of the metal. c Paste
polished titanium—a mechanically abrasive method that physically removes material from the
surface which is based on the relative hardness of the metal. Polishing results in an R
a
of
approximately 0.2-0.3 lm
method that involves submerging the implants in an electrolyte solution to which a
charge is applied. Material is removed from the surface at a rate that is dependent
on the electrical conductivity of the metal or its alloying elements. In contrast,
paste polishing is a mechanically abrasive method that physically removes surface
features. The removal of material is based on the relative hardness of the metal and
it alloying elements. Essentially, a 'hard' material will provide resistance to the
physical abrasion, which helps produce a homogenous smoothened surface
(Fig.
2
). Both these techniques were employed to reduce the surface micror-
oughness of titanium and two of its alloys, TAN and titanium-15% molybdenum.
It was found that compared with microrough control surfaces, polishing of tita-
nium and its alloys significantly increased the occurrence of soft tissue capsule
formation in hand fracture fixation devices in an in vivo rabbit model in the
tibia [
79
]. Furthermore, polishing significantly reduces the force required for
removal of conventional and locked screws as well as intramedullary nails from
sheep tibial bone after short-term (6, 12 and 18 weeks) and long-term (6, 12 and
18 months) implantation [
78
,
80
,
81
]. Histologically, it was observed that polished
implants supported fibro-osseointegration or the occurrence of a very thin fibrous
layer (sometimes only one to three cells thick) between the bone and implant
without loss of implant stability. These results also challenge, therefore, the
general notion that direct bone bonding is required for stable fixation (Figs.
3
,
4
).
3.3 Roughness Spectrum
Cell-material interaction studies have clearly defined that a microrough surface is
inductive for osteoblast differentiation, and that this phenomenon is echoed by
microrough implants in vivo. However, a clear definition describing what con-
stitutes a 'rough' or 'smooth' surface (millimetres, micrometres, nanometres) is
distinctly lacking. One of the main reasons for this omission is that different
Search WWH ::

Custom Search