Biomedical Engineering Reference
In-Depth Information
Step-addition (polycondensation) of BTA
-
DMG.
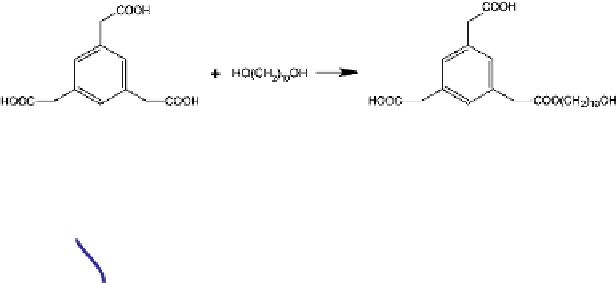
Figure 3.1
DMG
BTA
Potential cyclization
Graph-like representation of BTA
-
DMG step addition leading to gelation, showing a potential
intramolecular linking site.
Figure 3.2
benzene triacetic acid (f
n
= 3) and decamethylene glycol (f
m
=2)(BTA
-
DMG), which were
designed speci
cally as a model polyester system by separating the acid groups with a
benzene ring and the diol reactants by an extended chain of 10
-
CH
2
-
units. This system
has been studied by a number of groups.
Figure 3.1
shows the structure and chemistry of the BTA
DMG system, and
Figure 3.2
shows the same with the chemical detail reduced to ball and stick form. In this case,
where f
m
= 2 and f
n
= 3, according to Flory
-
s gel criterion, gelation should occur when p
c
=
(2)
−
1/2
= 0.707 (Stepto,
1998
). To quote Flory himself, this system
'
appears to be a
particularly felicitous choice in this respect, the vitiation of the gel point being less than
one percent
'
0.72 (Gordon
et al.,
1975
; Ross-Murphy,
1975
). The increase is due, in this case, to the presence of
c.1% of intramolecular polyester linkages
'
(Flory,
1974
), so that measurements typically give p
c
≈
0.71
-
most of which can be eliminated by
following the procedures introduced by Stockmayer and co-workers (Jacobson et al.,
1950
). They measured p
c
in the presence of various concentrations c of diluent, and then
extrapolated to 1/c = 0. In this way the value of p
c
found for the BTA
-
cycles
-
-
DMG system was
essentially the classical Flory value of 0.707.
3.2
Percolation model
At the end of the 1970s, physicists specializing in second-order phase transitions in the
vicinity of the critical point of liquid
-
vapour mixtures or of magnetic transitions came up




























































Search WWH ::

Custom Search