Biomedical Engineering Reference
In-Depth Information
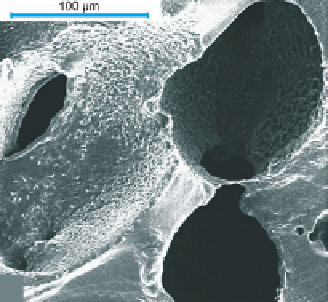
Figure 11.13
Scanning electron micrograph of a superporous hydrogel (SPH). Reproduced with permission from
Omidian et al.(
2005
) © 2005 Elsevier.
4. Foaming aid, foam stabilizer
3. Cross-linker
5. Oxidant
2. Neutralization
1. Monomer dilution
6. Reductant
7. Bicarbonate
SPH
Figure 11.14
Steps in the production of a superporous hydrogel (SPH). Reproduced with permission from
Omidian et al.(
2005
) © 2005 Elsevier.
added to generate gas bubbles. Dispersion and dissolution of HCO
3
−
increases the pH of
the reaction medium to a level at which the initiator decomposes faster. When the
formation of initiator radicals reaches a certain level, polymerization proceeds rapidly
and the reacting mixture becomes viscous over time. At the same time, HCO
3
−
reacts
with the acid component to produce the CO
2
required for the blowing process.
The two processes, i.e. the cross-linking reaction (gelation) and foaming processes, need
to be controlled so that 80% of the foaming develops before gelation. Then the rest of the
foaming occurs, the cross-linking reaction takes place and temperature increases by 5
10°C.
As cross-linking proceeds further and the temperature reaches its maximum, the foam turns
into a
-
flexible, rubber-like material. Then the synthesized foam is added to a non-solvent,
usually ethanol, in several batches until it is completely dehydrated, to stabilize the product
and prevent it from shrinking. This results in a solid, brittle, porous product which can be
ground into particles (like SAP particles), sliced into absorbent sheets or machined to a
desired shape and size. Dried SPHs swell fast in water and to a size a few hundred times their
















Search WWH ::

Custom Search