Biomedical Engineering Reference
In-Depth Information
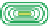
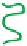
(A) Schematic representation of the neutralization steps for a polyelectrolyte alcohol gel and the
methodology for building a multi-membrane structure: (a) structure of alcohol polyelectrolyte gel;
(b) the neutralization step: chain condensation and shrinkage of the alcohol gel with the
disappearance of ionic repulsions; (c) formation of the interphase in solution; (d) formation of an
inter-membrane space outside the neutralization bath and complete retraction of residual polymer
chains from the interphase solution. (B) Overview of the multi-step neutralization process. Adapted
from Ladet et al.(
2008
) with permission from Nature Publishing Group.
Figure 11.5
a maximum, leading to more homogeneous gels. In contrast, for low NaOH, water
diffusion within the alcohol gel must be considered, and the neutralization route used to
generate novel concentric (onion-) shelled multi-membrane architectures.
During the neutralization step, if water diffusion is fast enough compared to ionic
neutralization kinetics, an interfacial solution is formed in which the polymer mobility is
high enough to allow disentanglement of the polymer chains and their
on
to the neutralized gel (
Figure 11.5
). In this way, formation of an inter-membrane space
was promoted by slowing down the neutralization, simply by removing the gel from the
neutralization bath and washing with water. According to Ladet et al., membrane
formation occurs in a three-step sequence: (i) generation of a water
'
condensation
'
alcohol interphase
solution between the neutralized and alcohol gels, (ii) disentanglement of chains located
-












































































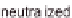























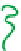
































































































































































































Search WWH ::

Custom Search