Biomedical Engineering Reference
In-Depth Information
(a)
X-EMG2
10
2
X-LBG
X-EMG1
10
1
X-GUAR
10
0
10
−
1
10
0
10
1
(b)
10
0
X-GUAR
X-EMG1
10
−
1
X-LBG
X-EMG2
10
−
1
10
0
10
1
(rad s
−
1
)
ω
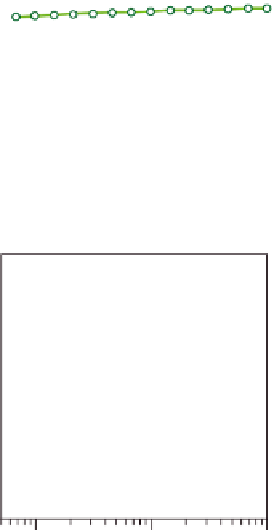
Figure 10.18
Shear modulus G
0
(a) and loss tan
δ
(b) as a function of frequency for blends of xanthan (X) with
native guar (Man/Gal = 1.48), EMG1(Man/Gal = 1.85), EMG2(Man/Gal = 2.86) and locust bean
gum (LBG) (Man/Gal = 2.82). Reprinted from Pai and Khan (
2002
) © 2002 Elsevier.
However, these explanations, although totally consistent, neglect any possible ther-
modynamic mechanism. For example, removing galactose side chains may lower the
'
ed samples, making them more susceptible to both
intramolecular and intermolecular aggregation without requiring any speci
solvent quality
'
for the EGM-modi
c molecular
mechanism. Richardson and Norton (
1998
) have induced
'
gelation
'
in LBG by using the
'
freeze/thaw
'
mechanism, which all suggests that polymer
-
polymer self-interactions are
favoured, and that a conventional
'
thermodynamic
'
approach, measuring, for example,
the second virial coef
cient by light scattering, might be fruitful.
Xanthan
konjac glucomannan
The above caveat being noted, X-ray
-
fibre diffraction measurements by Cairns et al.
(
1986
) helped to establish that intermolecular binding does occur in mixed KGM
-
xanthan gels. Dea et al.(
1977
) concluded that the interaction between KGM and xanthan
was stronger than that of LBG and xanthan, based on the observation that the melt
temperature of KGM
xanthan. But
Shatwell et al.(
1990c
) indicated that some thermoreversible gels were marginally
-
xanthan was nearly 20°C higher than that of LBG
-



















Search WWH ::

Custom Search