Biomedical Engineering Reference
In-Depth Information
10
2
10
2
10
1
10
1
10
0
10
0
10
−
1
10
-2
10
−
3
10
−
4
10
−
5
10
−
1
10
−
2
10
−
3
10
−
4
10
−
5
(a) 0
.
8% GELL alone
(b) GELL/KGM=0
.
4/0
.
4
10
−
2
10
−
1
10
0
10
1
10
−
2
10
−
1
10
0
10
1
10
2
10
2
10
1
10
1
10
0
10
0
10
−
1
10
−
2
10
−
3
10
−
4
10
−
5
10
−
1
10
−
2
10
−
3
10
−
4
10
−
5
(c) GELL/KGM=0
.
3/0
.
5
(d) 0
.
8% KGM alone
10
−
2
10
−
1
10
0
10
1
10
−
2
10
−
1
10
0
10
1
ω
(rad
s
−
1
)
Figure 10.16
Frequency dependence of storage and loss shear moduli, G
0
(open symbols) and G" (closed
symbols), for various mixing ratios of gellan (
'
GELL
'
) and KGM (total polysaccharide
concentration 0.8 wt%) at various temperatures, in the absence of cations: 30°C (
○
,
●
); 25°C
(
Δ
,
▲
); 15°C (
□
,
■
); 0°C (
♢
,
♦
). Reprinted with permission from Miyoshi et al.(
1996
) © 1996
Elsevier.
solutions formed a gel at any temperature. The authors concluded that KGM was
adsorbed on to the surface of large aggregates of gellan gum helices, and the interactions
between KGM and gellan gum were promoted with increasing concentration of cations.
Nishinari et al.(
1996
) suggested that in KGM
gellan gum mixtures, the main ordered
structures are formed by gellan molecules and that KGMmolecules inhibit gel formation
by gellan alone.
Mixtures of gellan
-
-
KGM showed a maximum synergy at the mixing ratio 3/5
(Miyoshi et al.,
1996
). On adding monovalent cations, the storage and loss moduli of
mixtures increased and the gelation temperature shifted to higher temperatures. Divalent
cations also promoted the gelation of the mixtures, but excessive addition of divalent
cations decreased the moduli, which was attributed to phase separation.
Gellan
xyloglucan
The mixture of gellan and xyloglucan was found by Nitta et al.(
2003
) to form a gel in a
concentration range where the individual polysaccharides do not. Both TSX and (Na) GG
(sodium form gellan gum) solutions behave as viscoelastic
-
fluids at low concentrations or
high temperatures. In the case of TSX solutions, G
0
was smaller than G
00
, and GG

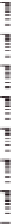

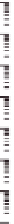

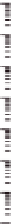

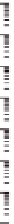






































































































































































Search WWH ::

Custom Search