Biomedical Engineering Reference
In-Depth Information
Opinions in the literature differ as to the driving force for the spontaneous gelation of
aqueous PVA solutions at room temperature. However, the role of crystallinity appears to
be widely accepted. For some authors (Ogasawara et al.,
1975
,
1976
; Watase and
Nishinari,
1985
;Wuet al.,
1991
), liquid
liquid phase separation is the primary event,
with crystallization in the polymer-rich phase reinforcing this domain as a secondary
effect, while others contend that junction zones directly crystallize from the solution
phase. Another opinion is that both conjectures are true, depending on gelation con-
ditions (Komatsu et al.,
1986
). The second hypothesis (direct crystallization) is supported
by essential observations from the literature. Water is known to be a good solvent for
PVA near and above room temperature, partially explaining why PVA melting temper-
atures are deeply depressed in the gel. PVA gels lose mechanical properties at 60
-
80°C,
roughly corresponding to the DSC endotherm associated with the melting of PVA
crystals. This superposition strongly suggests that crystallites hold the gel network
together.
The gelation of PVA solutions by freeze/thaw processing is represented schematically
in
Figure 8.19
(Willcox et al.,
2000
). After the
-
first temperature cycle, network formation
can be attributed to the formation of cross-links by kinetically frustrated crystallization.
As more freeze/thaw cycles are applied, or as the gel is aged, additional PVA crystal-
lization reinforces the original gel network. Reinforcement mainly involves the forma-
tion of a new, secondary class of crystallites, but the primary crystallites created in the
first quench may also grow. The average crystallite size remains small, in the range of a
few nanometres, and the gel mesh spacing of tens of nanometres changes little.
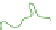
Figure 8.19e
also shows how EM imaging may change the real gel structure after removal
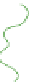
(a) Fresh solution
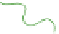
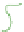
(b) Fresh
N
c
= 1 gel
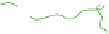
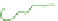
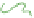
(c) Cycled / aged
TEM specimens
(d)
(e)
Schematic gel structure of PVA cryogels after various cycles: (a) fresh solution; (b) primary
crystallites appearing during the first temperature cycle; (c) secondary crystallites develop after
cycling/ageing; (d) TEM observations by removal of the frozen water in fresh gels; (e) TEM
obervations of cycled/aged gels with large pores, obtained after collapse of the amorphous chains.
Adapted from Willcox et al.(
2000
) with permission from John Wiley & Sons.
Figure 8.19

















































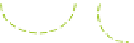


















































































































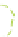
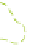






















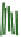










Search WWH ::

Custom Search