Biomedical Engineering Reference
In-Depth Information
7
CS
2
gels
6
5
4
3
2
−
2.0
−
1. 5
−
1. 0
log
T
/
T
gel
−
0.5
0
0.5
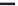
Figure 8.11
Master curve of aPS-CS
2
gel modulus versus temperature for various molecular masses and
concentrations. Reprinted with permission from Koltisko et al.(
1986
) © 1986 American Chemical
Society.
8.3.2.1
Rheology of transparent gels
Rheological properties were carefully measured by Koltisko et al.(
1986
). Narrow
polydispersity aPS (M
w
/M
n
< 1.2) samples with M
w
ranging from 2 to 900 kg mol
−
1
were investigated. Simple shear tests were performed on gels as a function of temperature
(between +25 and
100°C), molecular mass and concentration. Solutions were intro-
duced into a coaxial cylinder instrument and gels were formed in situ by lowering the
temperature of the apparatus. Stress
−
strain measurements were conducted by attaching
the inner cylinder to the crosshead of a standard Instron tensile testing device.
Measurements of the shear modulus G
eq
were performed by cooling the gel to the desired
temperature, allowing the gel to equilibrate for 10 min and applying the stress. The
modulus
-
temperature curves are similar in shape for all concentrations. As temperature
decreases below the gelation threshold temperature, the modulus increases over a 40°C
temperature range and then levels off at a value that depends on the concentration.
Molecular masses of 35 kg mol
−
1
and higher exhibit the same low-temperature plateau
modulus over which the gel modulus is constant. The width of the plateau depends on
molecular mass, and, like the rubbery plateau, as the molecular mass increases the
temperature range becomes broader. The data shown in
Figure 8.11
corresponds to
transparent gels, above the binodal line. It follows that the mechanism of gelation may
be similar to that describing the rubbery plateau in a temperature region over which
physical associations between chains are allowed to form.
The values of the modulus obtained with various reduced temperatures (T/T
gel
) and a
-
xed M
w
indicate that the modulus depends on the square of the concentration over the
entire temperature range. In order to construct the master curve in
Figure 8.11
, which
includes data for various concentrations (M
w
= 900 kg mol
−
1
), the shift factors for the
effective modulus G
eff
were de
ned by
2
;
G
0
c
G
eff
¼
ð
8
:
1
Þ



















Search WWH ::

Custom Search