Biomedical Engineering Reference
In-Depth Information
Percolation regime
10
4
10
3
Cod 4.5% g cm
−
3
Cod 8% g cm
−
3
Tuna 4.5% g cm
−
3
Tuna 8% g cm
−
3
A1 8% g cm
−
3
T
=
1. 2
C
T
=
0.8
°
C
T
=
10
°
10
2
°
C
T
=
10
C
T
=
10
°
C
°
10
Gelation thresold
1
0.00
0.01
0.02
0.03
0.04
0.05
c
helix
(g cm
−
3
)
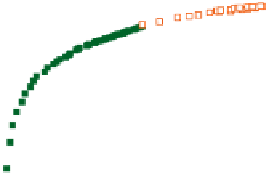
Figure 7.15
Master curve for the storage modulus of all types of gelatin networks. Adapted with permission
from Joly-Duhamel et al.(
2002a
) © 2002 American Chemical Society.
(cod and tuna), which gel at different concentrations or temperatures, for some samples at
temperatures as low as 1°C.
7.2.8
Elasticity in gelatin gels
If we consider the model of alternating
flexible and triple-helical segments along the
chain, then a
first approximation would be to assume that the very
flexible polypeptide
segments act like an ideal rubber, and in practice a good
fit can be obtained on this basis.
Alternatively, if we adopt the model of a proportion of rods in a sea of coils, then a rod-
like model may be more useful, as introduced below. Here we summarize both models.
7.2.8.1
Coil-like model
Using the information above and the branching theory described in
Chapter 3
,itis
possible to obtain a very good zero-parameter
fit to the modulus versus concentration
behaviour of gelatin, using creep data (equilibrium compliance, J
e
=1/G) to provide good
quality estimates (Higgs and Ross-Murphy,
1990
). As noted above, at the gel point the
helix fraction
χ
c
~ 0.07, for a gelatin concentration 4.5%w/v, so from the simple Flory gel
point (
Equation (3.3)
) this would give an apparent maximum number of branches per
chain (the functionality, f,asde
ned in
Chapter 3
)of
χ
c
= 1/(f
−
1), i.e. f ~15.
c model may be that of Peniche-Covas et al.(
1974
), which suggests
that for an n-chain model, f =(n
A more speci
1) + 1. Here, x is the number of junction zones
per chain and n (= 3 for gelatin) is the number of chains involved in this junction zone. We
can also estimate the functionality if we use the fact that the minimum stable helix length
is around 30 residues (Busnel et al.,
1989
). Since the molecular mass of a peptide residue
in gelatin is ~10
5
g mol
−
1
, this means that for this sample (M
n
~ 70 000 g mol
−
1
) the
maximum functionality per chain should be around 20, so that, for a three-chain triple
helix model, f ~ 40.
The latter estimate is almost certainly too high because of the presence of covalent
cross-links, the need for the network to retain
−
1)(x
−
flexible regions etc., so a
figure of f ~10
-
20




































Search WWH ::

Custom Search