Biomedical Engineering Reference
In-Depth Information
7.2.6.4
Helix thermal stability
The coil
helix transition is reversible, but there is an element of hysteresis between the
formation (renaturation) and melting (denaturation) temperatures, the extent of which
depends upon the relative heating and cooling rates. Moreover, while collagen denatura-
tion has a very narrow temperature distribution, the renaturated helices of gelatin gels
present a large distribution of melting temperatures, with a clear correlation between the
width of the melting peak and the helix formation temperature: after maturation, the
lowest temperatures provide the largest helix fractions, but the broadest distributions.
The maximum of the peak is also related to the
-
final cooling temperature, with the
lowest gelation temperatures having the lowest melting temperatures.
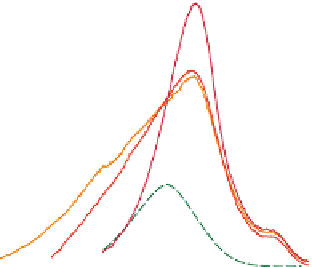
Figure 7.8
shows
0.06
(a)
A1
A2
0.05
0.04
0.03
0.02
0.01
0.00
10
15
20 25
Temperature (
°
C)
30
35
40
45
(b)
Mammalian collagen
40
35
30
25
A1
20
A2
15
10
5
0
0
5
10
15
20
25
30
35
40
45
Annealing temperature (
°
C)
Figure 7.8
(a) Melting curves d
χ
/dT for samples A1 and A2 after cooling and annealing for 15 h at various
temperatures, indicated by the arrows. The melting temperatures vary according to the initial
temperature and depend on the molecular mass. (b) Melting temperature versus annealing
temperature for samples A1 and A2. Extrapolation toward the melting temperature of
mammalian collagens is shown. From Joly-Duhamel et al.(
2002b
) © 2002 American Chemical
Society.





































































Search WWH ::

Custom Search