Biomedical Engineering Reference
In-Depth Information
T
T
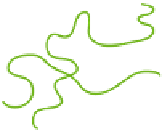
Schematic representation of the coil
-
triple helix transition for a semi-dilute gelatin solution.
Figure 7.4.
change of the coils in solution. Although many parameters can affect the thermal
'
renaturation
'
of triple helices, we examine below only one type of structure
-
the triple
helix conformation
chains can adopt.
The fact that triple helices do not associate in bundles was, at one time, controversial.
Godard et al.(
1978
) attributed the melting enthalpy of the gels during maturation to the
formation of fringed micelle crystals, an interpretation based on generally accepted ideas
for crystallization of polymer melts. Later measurements such as optical rotation demon-
strated that the mechanism of renaturation is limited to the triple helix step. Moreover,
X-ray
-
fibre diffraction experiments (Pezron et al.,
1990
) displayed scattering patterns close
to native collagen, but without features related to the macro-period of the
fibrils along the
longitudinal axis. In the rehydrated state, the orientation of the triple helices progressively
decreases with water content and the spacing between the triple helices increases, while the
positions of the meridional wide-angle re
ections (periodicity along the triple-helical
structure) are unchanged. These experiments support the assumption that gels which
usually contain large amounts of water (>50 wt%) have a network structure in which
segments of triple helices are randomly oriented. Moreover, since measurements of the OR
angle show a slow increase with time but apparently without limit (even when plotted on a
log time axis), the proportion of residues in the ordered helical conformation must also be
increasing. This suggests a considerable degree of conformational
flexibility, even post-gel,
and is rather unlikely to occur if the junction zones are formed of rigid crystallites.
7.2.6.1
Optical rotation (OR)
Conformational changes of certain biopolymer solutions can be detected by measuring
the rotation angle of the polarization of an incident linearly polarized light beam. Far
from the absorption band, the optical rotation angle changes as the beam passes through
the solution. The collagen triple helices have the same optical rotation properties as the
left-handed helices of the individual strands. The triple helix association gives a dis-
tortion to the chains which has very little effect on the optical rotation dispersion. Just as
for carrageenans (
Chapter 5
), for gelatin solutions and gels the change of optical rotation
angle can be related to the proportion of residues renatured or reassembled into helices
(hereafter referred to as the
'
helix fraction
'
):
ex
λ
- ½
coil
λ
½
χ ¼
;
collagen
λ
coil
λ
½
- ½

























Search WWH ::

Custom Search