Biomedical Engineering Reference
In-Depth Information
0.8
Na
+
, K
+
0.7
0.6
35
°
C
Na
+
γ
15
°
C
0.5
0.4
K
+
15
°
C
0.3
0.2
0.05
0.10
0.15
0.20
√
c
(eq/l)
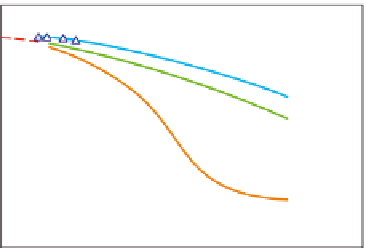
Activity coefficient of monovalent counterions (K
+
and Na
+
) as a function of polymer
concentration c at 15°C and 35°C. Reprinted with permission from Rochas and Rinaudo (
1980
)
© 1980 John Wiley & Sons.
Figure 5.11
γ
Na
+
was not so dependent on temperature in the
At higher polymer concentrations,
γ
K
+
was 0.37. Since
λ
P
)
−
1
for monovalent cations and
range 15°C to 35°C, while
γ
1
=(2
λ
P
)
−
1
γ
2
=(4
λ
P
is the so-called charge parameter (or the
inverse of the distance between two charged groups), the value of
for divalent cations, where
λ
P
was doubled. Also,
the melting enthalpy determined from the DSC endothermic peak in water is double the
value observed in non-aqueous solvents (formamide and DMSO) in which a coil-single
helix transition is thought to occur. With these results and the doubling of the molar mass
under non-gelling conditions, Rochas and Rinaudo (
1980
) concluded that double- rather
than single-helix formation occurs in water and electrolyte, and that now appears to be
the consensus.
For gellan, Morris, E. R. et al.(
1999
) reported that the shear storage modulus G
0
,
breaking stress
-
0.7%) as a function of NaCl showed a maximum. In other words, all parameters increased
and then decreased with increasing concentration of NaCl, with the maximum value
corresponding to a NaCl concentration of about 300mM for G
0
,and
σ
b
and gelation temperature T
gel
at a
fixed Na-gellan concentration (0.5
σ
b
,and750mMfor
T
gel
. The Young
s modulus and the breaking force of gellan gels as a function of potassium
chloride concentration showed a similar maximum (Milas and Rinaudo,
1996
). This
experimental
'
finding, that the elastic modulus of these polyelectrolyte gels as a function
of added salt shows a maximum, seems to be universal: the storage Young
'
smodulusof
κ
-carrageenan gels as a function of added alkali metal salts was also reported to show a
maximum (Watase and Nishinari,
1982
). Work by Kasapis et al.(
1999
)showedamax-
imum of the storage shear modulus of gellan gels as a function of added CaCl
2
and NaCl.
The increase in elastic modulus or transition temperature may be understood by the
shielding of electrostatic repulsion between, respectively,
the sulphate groups in
κ
-carrageenan or the carboxyl groups in gellan, helping promote helix formation, but
the reason for the subsequent decrease is not yet fully understood. On addition of excess
salt, other phenomena, such as phase separation, may occur and so
'
'
the overall
gel structure. Morris et al.(
1995
) explained this behaviour in terms of the transition from
weaken

































Search WWH ::

Custom Search