Biomedical Engineering Reference
In-Depth Information
Narrow signal
Broad signal
×
20
60
40
20
0
−
20
−
40
10
0
−
10
δ
(ppm)
δ
(ppm)
133
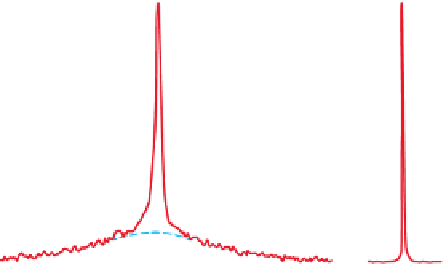
Cs NMR spectra of 3% w/v caesium
κ
-carrageenate in D
2
0 at 80°C (right) and 25°C (left)
obtained at 13 MHz (JEOL FX-100 NMR spectrometer). Chemical shift is positive downfield
relative to
133
Cs ions in 10 mM CsCl in D
2
O, contained in a central coaxial tube. Reprinted with
permission from Grasdalen and Smidsrød (
1981
) © 1981 American Chemical Society.
Figure 5.9
molecules around K
+
move faster than those around Na
+
, and that the bond length of
M
+
H
2
O(M
+
=K
+
,Cs
+
,Rb
+
,Cs
+
,Na
+
,Li
+
etc.) decreases in the order Cs
+
>K
+
>Na
+
>
Li
+
>NH
4
+
, based on the dynamic hydration number of these cations determined from
NMR, neutron scattering and simulations (Ohtaki and Radnai,
1993
).
Using
133
Cs NMR, Grasdalen and Smidsrød (
1981
) concluded that caesium ions bind
-
to
-carrageenan by observing the restriction on mobility which appears as a broadening
of the spectra for a 3% w/v solution of Cs
+
κ
-carrageenate in D
2
O as the temperature is
lowered from 80°C to 25°C. This clear broadening was not observed in
κ
-carrageenan,
where only a slight broadening, due to increased viscosity, was observed at low temper-
ature; see
Figures 5.9
and
5.10
.
A motional restriction of at least three orders of magnitude for bound ions relative to
free ions is indicative of strong and long-lived binding of the Cs
+
ions at well-de
ι
ned
sites on the carrageenan molecules. Grasdalen and Smidsrød (
1981
) argued that, in
addition to the sulphate groups, these sites consist of one or more groups with hydroxyl
and ring oxygens to stabilize the binding of the gel-promoting larger alkali metal ions,
and arranged to create a cavity. The temperature dependence of the
133
Cs NMR line shifts
for 3% w/v caesium
κ
-carrageenate in D
2
O on cooling and heating show a similar
behaviour to the sol
gel transition observed by rheology, including hysteresis, and was
attributed to gel formation steps, i.e. the formation of junctions zones, accompanied by
site binding of counterions.
The
133
Cs line shift as a function of concentration of Cs
+
-
κ
-carrageenate showed a
steep increase at 1% w/v, and the curve was sigmoidal, indicating the cooperative nature
of the sol
-
gel
transition. Smidsrød and co-workers also found that gelation of
κ
-carrageenan produces highly selective binding sites for alkali metal ions, in which
Cs
+
and K
+
ions bind much more strongly than Li
+
or Na
+
. The so-called binding
selectivity coef
cient value at low potassium contents was ~10 for
κ
-carrageenan,



















Search WWH ::

Custom Search