Biomedical Engineering Reference
In-Depth Information
10
0
10
−
1
10
−
2
10
−
3
10
−
4
3.1
3.2
3.3 3.4
T
m
−
1
(K
−
1
×
10
3
)
3.5
3.6
Dependence of the inverse of the temperature of conformational transition (T
m
−
1
) on counterion
activity: Li-gellan, LiCl (
▲
); Na-gellan, NaCl (+); K-gellan, KCl (
○
); Me
4
N-gellan, Me
4
N
+
Cl
−
(
Δ
); Ca-gellan, CaCl
2
(
□
); Mg-gellan, MgCl
2
(
■
). Reprinted with permission from Milas and
Rinaudo (
1996
) © 1996 Elsevier.
Figure 5.8
Rochas and Rinaudo (
1980
) found a linear relation between 1/T
m
, the inverse of the
melting temperature, and the logarithm of the total salt concentration cT.
T
. They found a
similar relation for gellan (Milas and Rinaudo,
1996
), as shown in
Figure 5.8
.
For gellan, determination of the activity of counterions gives information on the
conformation of the polymer in dilute solutions, in particular in determining whether
the polymer adopts a single- or a double-helical structure. For
-carrageenan, a similar
relation between the transition temperature and the total concentration of free counterions
was reported by Hugerth et al.(
1999
), based on
κ
fluorescence measurements, dynamic
viscometry and optical rotation.
5.4.3
Role of monovalent cations
Since the pioneering work of Hofmeister almost a century ago, it has been recognized
that simple salts may drastically alter the solubility and other solution properties of
macromolecules. In general, the relative effectiveness of various ions follows a well-
de
ned order (the lyotropic series), which extends to phenomena far removed from
colloid chemistry (such as the surface tension of salt solutions, the miscibility of liquids
and the kinetics of organic chemical reactions in solution). In the cation-induced gelation
of negatively charged polysaccharides, however, the normal lyotropic order may be
distorted or scrambled by selective interactions of the polymer and counterion.
In the carrageenan series, the cation requirements for gelation and adoption of the
underlying, ordered junction-zone geometry are dependent on the degree of sulphation of
the polymer. For example, K
+
ions are more effective than Ca
2+
in promoting gelation of
κ
-carrageenan, the order is reversed
(Payens and Snoeren,
1972
; Rinaudo et al.,
1979
; Morris and Belton,
1980
; Morris and
Norton,
1983
).
The cations K
+
,Cs
+
,Rb
+
and Cs
+
have a stronger gelation-enhancing effect than Na
+
and Li
+
, probably because of the difference in hydration. It has been reported that water
-carrageenan, whereas, for the more highly charged
ι



































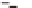






Search WWH ::

Custom Search