Biomedical Engineering Reference
In-Depth Information
interactions (see
Chapter 6
). As mentioned above, this usually involves some pre-
ordering of chains into more complex secondary structures.
The gelation of both
-carrageenan and deacylated gellan has been studied exten-
sively, and so is described in detail here. Helix formation is known to be a prerequisite for
gel formation for such anionic polysaccharides, including
κ
-carrageenan and
gellans (and also for some essentially neutral polymers such as gelatin and agarose; see
Chapter 7
). These biopolymers are known to be in a different, so-called
κ
- and
ι
'
random coil
conformation
at higher temperatures (although data for their persistence length in this
form is very limited), and do not form gels. At lower temperatures they undergo a
disorder
'
helix) transition and form aggregates in which it is now largely
accepted that shared helical portions form the cross-links of a three-dimensional network.
Since these physical cross-links have a
-
order (coil
-
finite size and so differ from the chemical point
cross-links in covalent gels, they are now all but universally referred to as junction zones.
In extensive studies of the optical rotatory power of polysaccharides in various
conformational states, Rees (
1969
) established a semi-empirical relationship between
torsion angles which speci
ed the relative orientations of residues in these saccharide
polymers, and the magnitude and sign of the optical rotation (Morris,
1994
). Applying
this relationship to data for cold-set gelled solutions of carrageenans, and in particular to
data on optical rotation versus temperature, Rees and co-workers established an initial
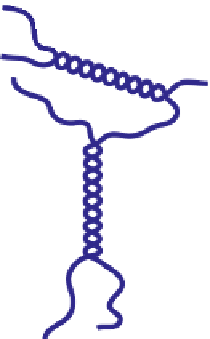
model for the cross-linking process; see
Figure 5.1
.
In this model, the cross-links were described as cooperatively formed junction zones
arising when regions of the carrageenan polymers combined to form ordered double
helices (and, in some cases, clusters of these helices). Cooperativity was inferred from the
suddenness of the optical rotation change in relation to temperature, and the presence of a
double-helical conformation was deduced by combining the lowest temperature optical
rotation results, and con
rmed with X-ray
fibre diffraction data.
Lower
t
emperatu
re
Add
ition of
ions
Schematic representation of the original gelation mechanism for
ι
- and
κ
-carrageenan. In the
middle, the chains are cross-linked by isolated double helices, while, on the right, double helices are
aggregated to an unknown extent. Reproduced from Rees (
1969
) by permission of the Royal
Society of Chemistry.
Figure 5.1




















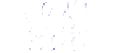



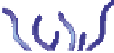



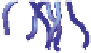





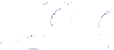
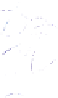
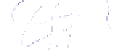



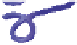


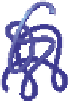



Search WWH ::

Custom Search