Biomedical Engineering Reference
In-Depth Information
50
Turbid
40
30
Sol
20
Hard gel
Soft
gel
10
0 0
20
30
40
50
60
70
c
(wt %)
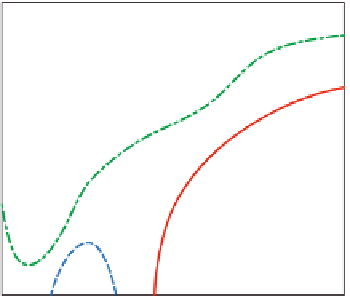
Phase diagram of aqueous solutions of S
5
E
45
S
5
. Open symbols: tube inversion; solid symbols:
rheology. Adapted from Ricardo et al. (2004) © 2012 American Chemical Society.
Figure 4.11
the authors predict the compressional and shear moduli. The moduli vary with different
power laws for (a) the aggregation number in the micelles, (b) the polymer chain length
and (c) the degree of compression X=(c
m
/c
min
−
1).
Experimental work on telechelic polymers and other associative type polymers is
presented in detail in
Chapter 6
. Light and neutron scattering, rheological experiments
and optical observations clearly show the tendency for phase separation of the telechelic
polymers and the formation of networks of micelles. As an example,
Figure 4.11
shows
results for solutions of a triblock copolymer of ethylene oxide and styrene oxide,
S
5
E
45
S
5
, where E denotes (OCH
2
CH
2
) and S denotes (OCH
2
CH(C
6
H
5
)) (Ricardo
et al.,
2004
). The phase separation corresponds to the cloudy region. It starts around
room temperature and appears when the sample is heated. In the homogeneous phase, at
increasing polymer concentrations one observes various states of aggregation from soft
gels (micellar liquids) to hard gels (closely packed micelles).
4.4.2.2
Zero-shear viscosity (SJK model)
Zero-shear viscosity was calculated for various concentration regimes using (
4.10
). Since
this is the product of the shear modulus and the characteristic time of dissociation of the
micelles, it is important to determine the characteristic relaxation times in the various
states, according to c
m
.
If the fraction of bridges is small (c
m
<< c
min
), the authors show that the characteristic
time of relaxation is the time of transformation of a loop into a bridge
τ
1
or back, and is
controlled by the expulsion of one adsorbing end group from the core of the micelle, as in
(
4.11
) of the TE model:
τ
1
~
τ
0
exp
ð
B
=
k
B
T
Þ;
ð
4
:
18
Þ
τ
0
is the microscopic exchange time for an end group, determined by its size and
the solvent viscosity.
where

















































Search WWH ::

Custom Search