Biomedical Engineering Reference
In-Depth Information
I
Collagen triple helix
II
Collagen + Ca ions
III
Collagen fibril + Ca ions
Mineralized collagen fiber
VI
Mineralized collagen fibril
(collagen fibril + HA)
V
Collagen fibril + ACP
IV
Polycrystal of nano-HA
ACP
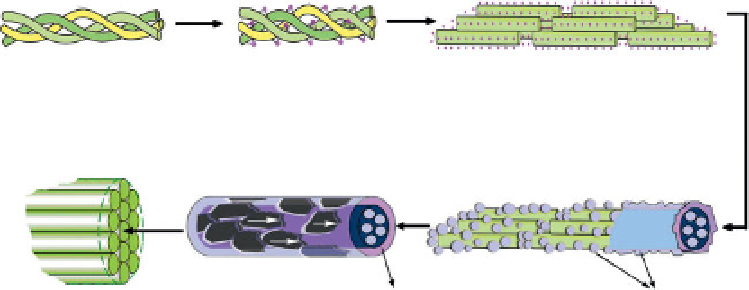
Figure 2.8
The hierarchical structure of a self-assembled HA-collagen composite.
I: collagen triple helix molecule. II: collagen molecule combined with Ca2
+
.
III: collagen fi bril combined with Ca2
+
. IV: collagen fi brils with ACP. V: the
organization of collagen fi brils with respect to HA crystals. VI: the organization
of the mineralized collagen fi ber.
According to the experimental results, they concluded the key mecha-
nism behind how these mineralized collagen fi brils self-assembled. The
hierarchical assembly of the specifi c mineralized fi bers occurs in several
stages, as shown in Figure 2.8. Firstly, triple-helical collagen molecules
chelate Ca
2+
through carboxyl and carbonyl groups and self-assemble
into fi brils about 5 nm wide. In the second stage, ACP associated with the
fi brils in their hole zones and the surface of collagen begin to form. And
then amorphous/crystalline conversion happens forming HA crystals on
the surface of collagen with their axes orienting along the long axes of
the collagen fi brils. The diameters of the mineralized collagen fi brils are
around 5.5-6.9 nm. Finally, the mineralized collagen fi brils organize paral-
lelly to form mineralized collagen fi bers with 77-192 nm wide.
These fundamental studies provide the basic theoretical support for the
fabrication of HA/collagen composites and their application in bone regen-
eration [63, 64]. Moreover, the development of novel self-assembled struc-
tures should therefore improve our understanding of collagen-mediated
mineralization in calcifi ed tissues, and point the way to the development
of new functional materials for biomimetic engineering.
2.3.2
In Vitro
Self-Assembly of Mineralized Recombinant
Collagen Fibrils
The recent development of recombinant protein expression technology
provides a reliable, predictable and chemically defi ned source of puri-
fi ed human-like collagen polypeptide that is free of animal components.
Recombinant human-like collagen (RHLC) has good prospects in tissue
Search WWH ::

Custom Search