Biomedical Engineering Reference
In-Depth Information
(ATP)-loaded MSN delivery systems by using disulfi de bond-reducing
molecules, such as dithiothreitol (DTT) and mercaptoethanol (ME), as
release triggers [40]. The biocompatibility and delivery effi ciency of the
MSN system with neuroglial cells (astrocytes)
in vitro
were demonstrated.
In contrast to many current delivery systems, the molecules of inter-
est were encapsulated inside the porous framework of the MSN, not by
adsorption or sol-gel types of entrapment, but by capping the openings of
the mesoporous channels with size-defi ned CdS nanoparticles to physi-
cally block the drugs/neurotransmitters of certain sizes from leaching out.
It can be envisioned that this new MSN system could play a signifi cant
role in developing new generations of site-selective, controlled-release
(
a
)
Cl
N
Si
OH
OEt
N
N
EtO
N
MCM-41
OEt
O
N
Si
O
DMF /TEA
353K
Toluene
reflux
O
O
HN
Si
HO
OEt
OEt
O
OH
EtO
7
MBi
MBI modified
MCM-41 MSNP
β
-Cyclodextrin
Cargo
β
-CD
Acid
(
b
)
(
c
)
HN
+
HN
+
H
+
N
N
Si
Si
Si
O
O
O
O
O
O
O
O
O
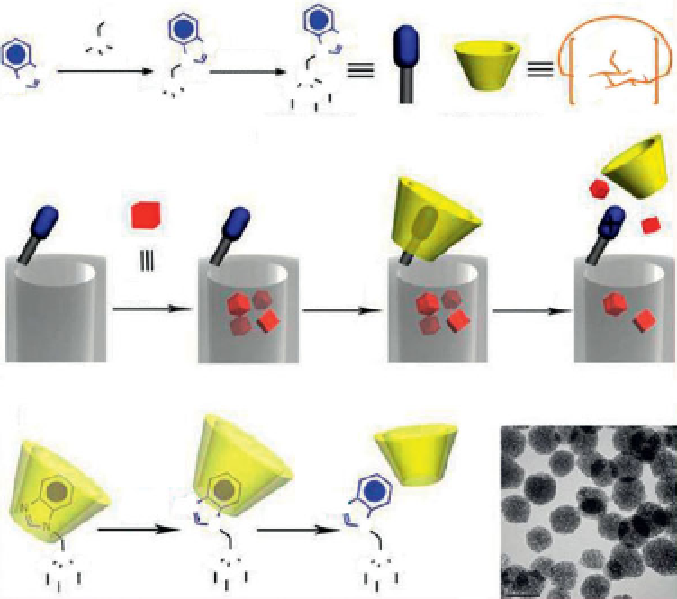
Figure 13.4
A schematic representation of the pH responsive MSNP nanovalve.
(a) Synthesis of the stalk, loading of the cargo, capping of the pore, and release
of the cap under acidic conditions. Based on our calculations, the maximum
number of stalks per nanopore is 6, and the maximum number of fully
assembled nanovalves per nanopore is 4. The average nanopore diameter of
the MSNP is around 2.2 nm, and the periphery diameter of the secondary side
of
b
-cyclodextrin is
1.5 nm. Thus, for a cargo with diameter >0.7 nm, a single
nanovalve should be adequate to achieve effective pH-modulated release.
(b) Details of the protonation of the stalk and release of the
b
-cyclodextrin.
(c) TEM image of capped MSNP. The scale bar is 100 nm. (Reprinted with
permission from [41])
∼
Search WWH ::

Custom Search