Biomedical Engineering Reference
In-Depth Information
amount of time before implantation, allowing more time for the scaffold
to degrade and cartilage to be formed (Figure 1.1).
1.3
Designing Scaffold for Cartilage Tissue
Engineering
Scaffold should be designed to provide a 3D environment which is suitable
for cartilaginous tissue production. In an ideal case the scaffold should have
a number of essential characteristics which are as follows: (i) promote cells
attachment, proliferation, viability and differentiation, and ECM production,
(ii) allow diffusion of nutrients and waste products, (iii) adhere and integrate
with the surrounding native cartilage, (iv) provide suffi cient mechanical integ-
rity depending on the defect location, and, fi nally, (v) controlled degradation.
Several researchers [9, 10] have found that the scaffolds degradation plays
an important role in cartilage regeneration. Scaffold degradation can occur
hydrolytically or enzymatically, and by controlling degradation tempo-
rally and spatially, scaffolds can accelerate and direct new tissue growth
[9, 10]. Degradable scaffold has improved ECM distribution compared to
completely non-degradable ones [9], and scaffolds with a slower degrada-
tion rate yielded cartilage of greater thickness in an osteochondral defect
model as reported by Solchaga and coworkers [10].
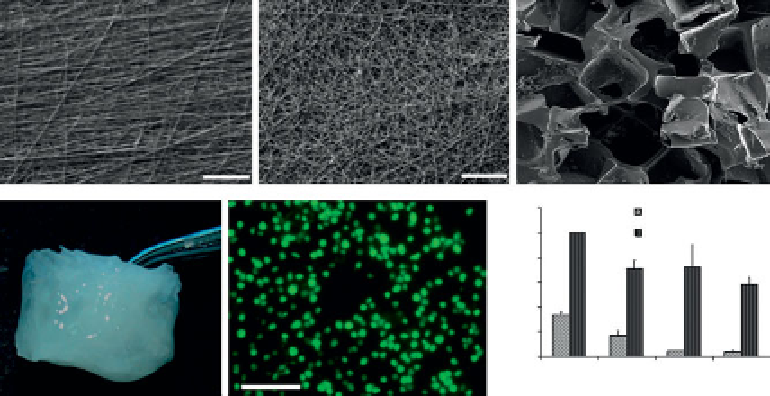
Three different forms of polymeric scaffold, namely, hydrogels, sponges
and fi brous meshes (Figure 1.2), have been fabricated and investigated
(
a
)
(
b
)
(
c
)
1.2
No TGF-
3
With TGF-
β
(
d
)
(
e
)
(
f
)
1.0
β
3
0.8
0.6
0.4
0.2
0.0
7
14
21
28
100
μ
m
Time (day)
Figure 1.2
Representative examples of different forms of scaffolds utilized for
cartilage tissue engineering. SEM images of (a, b) aligned and unaligned fi brous
scaffolds, (c) sponge, (d) hydrogel scaffold, (e) skeletal cells growth (rounded cell
morphology) within hydrogel, and (f)
col2a1
gene expression. Reprinted with
permission from [6, 7, 12]
.
Search WWH ::

Custom Search