Biomedical Engineering Reference
In-Depth Information
of an end unit, forming unstable 2,5-anhydro-D-mannose. Therefore, this
unstable end unit was further reduced to stable 2,5-anhydro-
D
-mannitol
by treating with NaBH
4
. After neutralization, the degraded chitosan was
fi ltered and concentrated by evaporation. Methanol was added to induce
precipitation with stirring and stored at 4
C to increase the precipitation
yield. After washing, the LMW-CS powders were dissolved in deionized
water and subjected to ultrafi ltration. Activated 4-azidobenzoic acid was
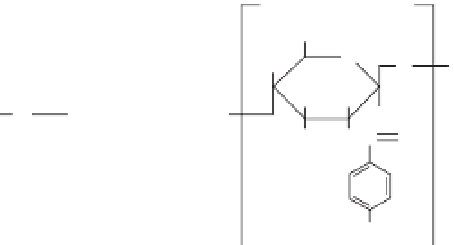
coupled to the LMW-CS, as shown in Figure 11.8a.
To prepare low molecular weight O-carboxymethyl depolymerized
chitosan (LMW-O-CMCS), the LMW-CS was dissolved in 60% NaOH
solution containing 0.2% dodecyl sodium sulfate and kept on ice for 1 h
until frozen. The frozen sample was suspended in isopropanol and mixed
with monochloroacetic acid. After the sample had been precipitated with
ethanol, the product was vacuum dried at room temperature. Finally,
the chitosan derivative was coupled with activated 4-azidobenzoic acid,
as shown in Figure 11.8b. Both of the azidophenyl chitosan derivatives
were noncytotoxic on the proliferation of mouse embryonic fi broblast 3T3
°
LMW-CS
Azido Benzoyloxy Succinimide
O
CH
2
OH
O
O
O
+
N
3
O
N
O
HO
NH
2
O
CH
2
OH
(
a
)
O
O
HO
NH
N
3
O
O
LMW-O-CMCS
CH
2
OCH
2
COOH
CH
2
OCH
2
COOH
O
H
O
CH
2
OCH
2
COOH
H
OH
O
H
O
H
(
b
)
NCH
O
H
OH
H
H
H
NH
2
n
N
3
n
Figure 11.8
Azidophenyl-derivatized (a) low molecular weight chitosan
(LMW-CS) and (b) low molecular weight O-carboxymethyl chitosan
(LMW-O-CMCS).




Search WWH ::

Custom Search