Biomedical Engineering Reference
In-Depth Information
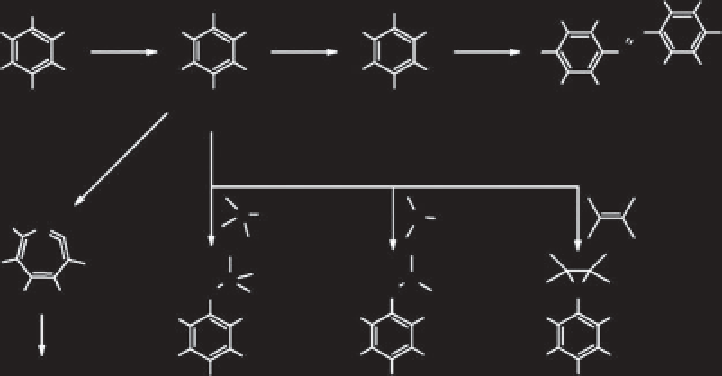
been synthesized by coupling with azidophenyl groups. These are decom-
posed by UV irradiation, and radical nitrene groups are produced by this
decomposition. The nitrene groups contribute to crosslinking of the poly-
mers with each other, and at the same time, they react with other organic
materials. Some recent developments have been described by Liu and
Yan [1], and the photochemistry is shown in Figure 11.3. The biopolymers
can be immobilized on substrata and employed for the immobilization of
organic materials including biomolecules such as nucleic acids or proteins.
We have utilized the biopolymers for fabrication of a micropatterned cell
culture substrate, immobilization of growth factors, and making micro-
array chips. The surface treatment is very important for biomaterial
design.
11.2.1 Gelatin
Collagen, fi bronectin, and vitronectin are important adhesion proteins.
Gelatin is produced from collagen and is used widely to enhance cell
adhesive properties. Matsuda and Sugawara [2] developed a microchemi-
cal fi xation method using azidophenyl functional groups. Azidophenyl
..
..
X
X
1
3
N
3
N
X
X
X
X
X
X
X
X
R
hv
N
III
R
N
-N
2
ISC
X
X
X
X
X
X
X
X
X
X
R
R
R
X = H, F
I
II
C
H
N
H
X
N
X
X
N
C
HN
N
HN
X
R
X
X
X
X
X
X
X
X
X
X
X
X
tar
R
R
R
Figure 11.3
Simplifi ed description of azidophenyl photochemistry.
Rearrangement of the corresponding seven-membered ketenimine, which reacts
with amines to give azepinamines, or produces polymer tars in the absence of
the nucleophile (I). CH or NH insertion and C
C addition reactions are the key
contributions to the covalent bond formation with the target molecules (II) and
relaxation via intersystem crossing to the triplet phenylnitrene, which undergoes
H-abstraction reactions to form primarily aniline-type products, or bimolecular
reactions to yield the corresponding azo compound (III). hv and ISC mean
photoirradiation and intersystem crossing, respectively. Reprinted and adapted
with permission from Ref. [1]. Copyright (2010) American Chemical Society.
=
Search WWH ::

Custom Search