Biomedical Engineering Reference
In-Depth Information
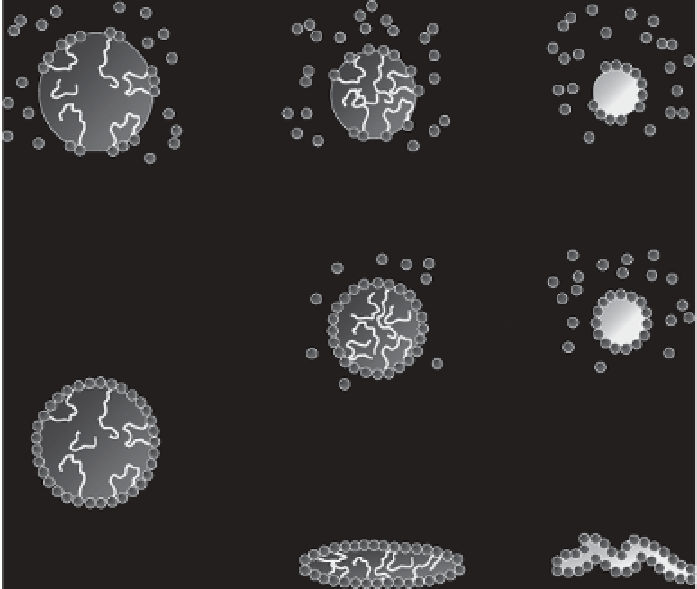
the molecular weight of 6,700 system, and the PS microsphere surfaces
were densely coated with the HAp nanoparticles. These results indicated
that deformation of droplets/microspheres is infl uenced by the extent of
the interaction between the HAp nanoparticles and polymer at the oil-
water interface (Figure 9.5). When weak interactions are involved (in
the case of PS-COOH with the molecular weight of 64,500, the number
of interaction points between PS-COOH and HAp at oil-water interface
is small), nanoparticles can desorb from the interface, and microspheres
shrink while maintaining a spherical form due to interfacial tension. In
contrast, when strong interactions (in the case of PS-COOH with the
molecular weight of 6,700, the number of interaction points is large) pre-
vent nanoparticles from desorbing from the interface, microspheres must
CH
2
Cl
2
evaporation
HAp
CH
2
Cl
2
Spherical
microsphere
Polymer
Surface area of droplet: decrease
Incomplete
surface coverage
Weak
interaction
between
HAp/Polymer
HAp: desorption
Surface area of droplet: decrease
Spherical
microsphere
Strong
interaction
Complete
surface coverage
HAp: remained
Surface area of droplet: constant
Collapsed
microsphere
Figure 9.5
Schematic representation of morphological changes of the droplets/
microspheres during dichloromethane evaporation. The strength of the
interaction between polymer and HAp is infl uenced by the polymer structure
(end groups and molecular weight) and the polymer concentration. Reprinted
with permission from [51]. Copyright © 2012 American Chemical Society.






Search WWH ::

Custom Search