Biomedical Engineering Reference
In-Depth Information
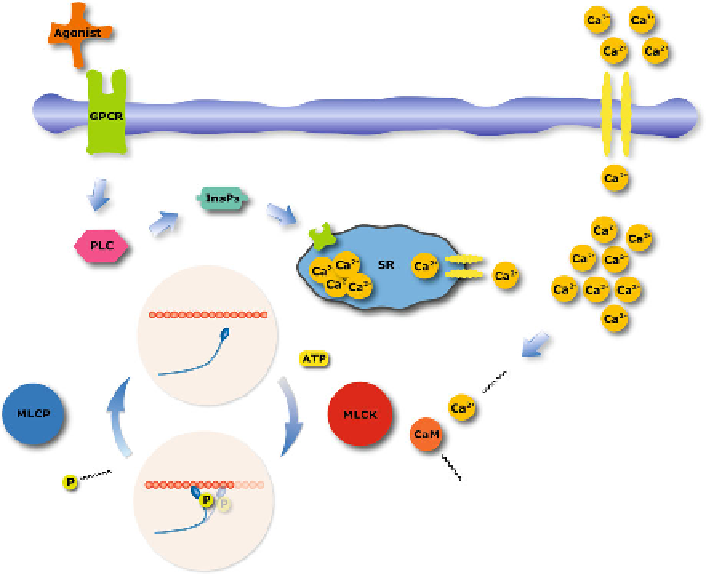
Fig. 4.1
Signaling pathways for Ca
2
+
-regulated myosin phosphorylation. Myosin phosphoryla-
tion/dephosphorylation is regulated by myosin light-chain kinase (MLCK) and myosin light-chain
phosphatase (MLCP). MLCK is activated by a calmodulin (CaM)-Ca
2
+
complex which depen-
dents on the level of intracellular
[
Ca
2
+
]
. The intracellular Ca
2
+
may be influenced in differ-
ent ways. Membrane depolarization and agonist stimulation through G protein coupled receptors
(GPCR); during membrane depolarization certain channels on the cell membrane open resulting
in an influx of Ca
2
+
. During agonist stimulation the GPCR activate phospholipase (PLC), which
induces inositol 1,4,5-triphosphate (InsP
3
) production and releases Ca
2
+
from the sarcoplasmic
reticulum (SR)
through ATP hydrolysis, similar as in skeletal muscle, causing muscle contraction.
The MLCP activity, which governs the dephosphorylation of the myosin regulatory
light-chains, has an effect on the Ca
2
+
sensitivity for the MRLC phosphorylation.
There are several pathways inhibiting the MLCP, such as Rho-Rho kinase and pro-
tein kinase C. However, here we are not considering variations in MLCP activity.
Smooth muscle is able to maintain active tension while the myosin phosphorylation
decreases. An explanation for this phenomenon was hypothesized by introducing an
attached, non-cycling (or slow-cycling), dephosphorylated cross-bridge (also known
as latch-bridge or latched cross-bridge), see Dillon et al. (
1981
). The introduction
of such as latched cross-bridge also explains the different contractile behaviors ob-
served for isotonic quick-releases performed at different time after isometric activa-
tion (Dillon et al.,
1981
).