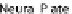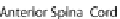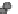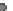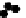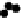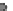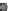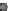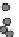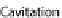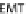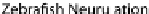Biomedical Engineering Reference
In-Depth Information
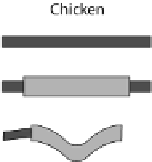
Fig. 24.1
Neurulation mechanisms. (
A
) Primary neurulation in the chicken. A central region
of ectoderm (neural plate) bends to form the neural groove. Multiple (brain) or single hinge
points (spinal cord) facilitate subsequent tube closure (
asterisks
). (
B
) Secondary neurulation in
the chicken. Mesenchymal cells coalesce and cavitate to form the posterior spinal cord. (
C
)Neu-
rulation in zebrafish. Cells migrate medially (
arrows
) to form the neural keel and reorganize to
form a slit-like lumen. (
D
) Schematic from Schoenwolf and Smith (
1990
) showing representative
cell morphologies during stages of hinge point formation in the prospective chicken brain, adapted
with permission from Development. Interrelated processes of cell shape change, contraction at the
apical (inner) wall, and nuclear positioning cooperatively shape the bending neuroepithelium
neurulation (Lowery and Sive,
2004
; Harrington et al.,
2009
). Hence, neurulation in
these species may involve a combination of the primary and secondary neurulation
mechanisms. Computational models for neural tube closure in amphibians have pro-
vided insight into some of these processes (Clausi and Brodland,
1993
; Chen and
Brodland,
2008
; Brodland et al.,
2010
).
What does seem to be clear, however, is that hinge points do not form during neu-
ral tube formation in
Xenopus
or zebrafish as occurs in chicken, mouse, and human
embryos (Fig.
24.1
A, C, Harrington et al.,
2009
). Hinge point formation is char-
acterized by interrelated, intrinsic processes such as cell wedging, possibly caused
by apical contraction or the radial positioning of nuclei in the neuroepithelial wall
(interkinetic nuclear migration) (Fig.
24.1
D, Schoenwolf and Smith,
1990
). The nu-
cleus constitutes the bulk of the cell volume (Fig.
24.1
D) and its radial position in
the neuroepithelial wall depends on the stage of the cell cycle. If, for example, a
subset of cells takes longer to undergo DNA synthesis at the outer wall of the neu-
roepithelium, then the nucleus would force the basal side of these tall, thin cells to
expand and potentially generate a hinge point (Smith and Schoenwolf,
1988
). Api-
cal narrowing via contraction may also be involved, however, as proteins that regu-
late cytoskeletal contraction (rho, phosphorylated myosin light chain, and F-actin)