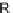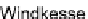Biomedical Engineering Reference
In-Depth Information
Fig. 15.2
Various
lumped-parameter models
utilized to specify boundary
conditions for the 3D
computational model. The
Windkessel model was
applied at all outlets except
the coronary tree; the heart
model was applied at the
aortic root. From Coogan et
al. (
2012
)
15.2.3.1 Heart Model
Overall function of the heart was simulated using a lumped parameter circuit model
(Kim et al.,
2009a
) that includes resistors and inductors to represent the mitral
and aortic valves (
R
AV
,
R
V
-
Art
,
L
AV
,
L
V
-
Art
), a pressure source that represents left
atrial pressure
P
LA
, and a variable capacitance that represents left ventricular elas-
tance/contractility
E(t)
, see Fig.
15.2
. The initial left atrial pressure was assumed to
be 10 mmHg. The final heart model parameters were
P
LA
=
11 mmHg, maximum
elastance of 1.25 mmHg/mL, and time-to-peak elastance of 0.4 s. A Lagrange pro-
file constraint with a penalty number of 10,000 was used to stabilize the solution
during the systolic phase of the cardiac cycle (Kim et al.,
2009b
).
15.2.3.2 Windkessel RCR Model
Hemodynamic conditions were prescribed at every outlet in the descending aorta,
neck, and head vessels in terms of a proximal (larger arteries and arterioles) resis-
tance
R
p
, a proximal vessel capacitance
C
, and a distal (small arterioles and capil-
laries) resistance
R
d
(cf. Fig.
15.2
). A Lagrange profile constraint was used at the
inlet and outlet of the aorta as well as at the outlets of the right and left subclavian
and external carotid arteries. Such constraints stabilize the computed solution while
affecting only the hemodynamics in a small region near the constraint (Kim et al.,
2009b
).