Biomedical Engineering Reference
In-Depth Information
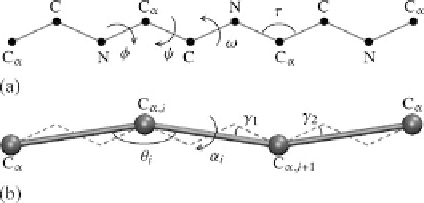
Fig. 1.1
All atom schematic of a polypeptide chain (
a
) and coarse-grained representation (
b
)of
the backbone with pseudo-bending and torsion angles. Side chains are not shown in (
a
). In (
b
)the
dashed lines
represent the polypeptide chain and the bond angle
θ
and dihedral angle
α
represent
the pseudo-bonds in the coarse-grained geometry
that
φ
and
ψ
dihedral angles are the only degrees of freedom of the all-atom back-
bone.
Figure
1.1
(b) demonstrates the CG chain by connecting the
α
-carbons through
pseudo-bonds. Since the all-atom bond lengths, bond angles and dihedral angle
ω
are not supposed to change, the pseudo-bond lengths between subsequent C
α
's re-
main fixed at a distance of 0.38 nm. Also, the pseudo-bending angle
θ
and pseudo-
dihedral angles
α
for the CG chain are defined between three and four consecutive
C
α
's, respectively. A geometrical expression can be established for the relation-
ship between the CG
(θ,α)
and all-atom
(φ,ψ)
degrees of freedom as suggested by
(Levitt,
1976
; Tozzini et al.,
2006
). The pseudo-bending angle for the coarse-grained
chain is given by
cos
θ
i
=
cos
τ(
cos
γ
1
cos
γ
2
−
sin
γ
1
sin
γ
2
cos
φ
i
cos
ψ
i
)
+
sin
τ(
cos
ψ
i
sin
γ
1
cos
γ
2
+
cos
φ
i
cos
γ
1
sin
γ
2
)
+
sin
γ
1
sin
γ
2
sin
φ
i
sin
ψ
i
,
(1.1)
14
.
7
◦
are constant angles (see Fig.
1.1
(b)). The exact
formula for the pseudo-torsion angle is very complex, but the following approximate
formula has been suggested by Tozzini et al. (
2006
):
20
.
7
◦
, and
γ
2
=
where
γ
1
=
α
i
=
180
+
φ
i
+
ψ
i
+
1
+
γ
1
sin
ψ
i
+
1
+
γ
2
sin
φ
i
.
(1.2)
It can be inferred from these equations that the pseudo-bending angle
θ
i
de-
pends only on one set of backbone dihedral angles
(φ
i
,ψ
i
)
, but the pseudo-
torsion angle
α
i
is a function of two consecutive sets of backbone dihedral angles
(φ
i
,ψ
i
,φ
i
+
1
,ψ
i
+
1
)
. It is worth noting that, in the force-fields developed specif-
ically for well-defined secondary structures, the simplifying assumptions
(φ
i
=
φ
i
+
1
,ψ
i
=
ψ
i
+
1
)
are made for mapping
α
(Levitt,
1976
; Tozzini et al.,
2006
). How-
ever, this assumption does not hold for proteins without any regular structure.