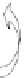Biomedical Engineering Reference
In-Depth Information
and operated at rotational speeds of w25 rpm. The low-
shear bioreactor summary is given in
Table 7.2-6 [18]
.
The time-averaged gravitational vector acting on these
cellular assemblies is reduced to about 10
2
g. In a typical
experiment on board a space craft or the space station
cycling in near-earth orbit, the gravitational force is ap-
proximately 10
4
-10
6
g.
Cell culture conditions in the ''simulated micrograv-
ity'' environment of rotating-wall bioreactors combine
two beneficial factors: low shear stress, which promotes
close apposition (spatial co-location) of the cells; and
randomized gravitational vectors. Close apposition of
the cells in the absence of shear forces presumably pro-
motes cell-cell contacts and the initiation of differen-
tiative cellular signaling via specialized cell adhesion
molecules. This process then might lead to the rapid
establishment and expansion of aggregate cultures which,
unlike in conventional bioreactors, are not disrupted by
shear forces. In addition, the low-shear environment, in
concert with randomized gravitational vectors, might
restrict diffusion of mitogenic and differentiative growth
factors, which are secreted by the cells. These autocrine/
paracrine feedback mechanisms might further enhance
the aggregation and differentiation, and contribute to the
observed capability of this environment to maintain high-
density cell cultures. Cultures in a 3-D matrix or scaf-
folds such as collagen and agarose gel largely maintain
the chondrocyte phenotype, but the use of RWVs as
scaffold-free bioreactors also leads to successful differ-
entiation and the hyaline production. The most signifi-
cant advantages of the RWV for a 3-D tissue culture are
low shear forces, the reduced risk of cell damage, and the
increased opportunity for cell-cell interaction. Thus,
primarily dissociated cells can reassemble to form a 3-D
tissue-like mass. The spatial orientation may be critical to
the differentiation of growing aggregate cells and to the
regeneration of a functional ECM.
Buoyancy
Medium flow
Gravity
Fig. 7.2-23 Rotating-wall vessel bioreactor.
Rotating-wall bioreactors are horizontally rotated with
fluid-filled culture vessels equipped with membrane
diffusion gas exchange to optimize gas/oxygen supply.
The initial rotational speed is adjusted so that the culture
medium, the individual cells, and pre-aggregated cell
constructs or tissue fragments rotate synchronously with
the vessel, thus providing for an efficient low-shear mass
transfer of nutrients and wastes. As the cell aggregates
grow in size and exhibit increasing sedimentation veloc-
ities, the shear stress can approach up to 0.5 dyn/cm
2
.
These simulated microgravity conditions facilitate spatial
co-location and 3-D assembly of individual cells into large
aggregates. There is laminar flow of medium over the
surface of constructs with fairly uniform shear stress
distribution. For example, the maximum shear stress on
the surface of constructs is approximately 0.3 dyn/cm
2
for a 120-ml rotating vessel containing only one construct
Table 7.2-6 Low-shear bioreactor summary
First author
Proteoglycan
Collagen
Additional notes
Gooch (construct, 2- to 4-week
bovine knee, 76 10
6
cells/cm
3
PGA, 4 weeks)
2.9-fold increase in GAG
composition
1.6-fold increase in collagen
composition
Supplemented with IGF-I
Martin (construct, 2- to 3-week
bovine knee, 127 10
6
cells/cm
3
PGA, 6 weeks)
75% increase in GAG
composition
39% increase in collagen
composition
Equilibrium modulus and GAG
composition reach native
cartilage levels after 7 months
in vitro
Freed (construct, 2- to 3-week
bovine knee, 127 10
6
cells/cm
3
PGA, 6 weeks)
68% of native GAG levels per
gram wet weight
33% of native type II collagen
levels per gram wet weight
Type II collagen crosslinked
in constructs
Refer to the original work for the references.