Biomedical Engineering Reference
In-Depth Information
terms of risk management, regulatory authorities would
prefer that cells cultured for clinical use avoid the use of
bovine and other animal-derived products, to reduce
the risk of disease transmission. Thus, a ''holy grail'' of
cell culture for clinical use is to develop an entirely
defined culture system. The use of autologous human
serum in tissue engineering is increasing.
Cell growth medium often contains supplements,
growth factors, and serum that may have an unknown
influence on cell adhesion and proliferation. For example,
the inclusion of the fibroblast feeder layer for keratino-
cyte expansion comes from the well-documented de-
pendence of ECs on mesenchymal cell interactions
between epidermal and dermal cells via soluble
factors providing important signals in regulating the re-
epithelialization of wound skin. Keratinocytes regulate
the expression of keratinocyte growth factor (KGF) in
fibroblasts through the release of interleukin-1b (IL-1b),
a proinflammatory molecule that increases fibroblast
proliferation and ECM production. The effect of solid
substrate on the paracrine relationship between kerati-
nocytes and fibroblasts as modulated by KGF and IL-1b is
unclear.
Although the significance of breathing ambient air
(containing 21% oxygen) to our survival is obvious,
physiologic ''normoxia'' is much lower. Therefore, the
traditional paradigm of culturing cells in humidified
ambient air may not be optimal for maintaining certain
cell types, including stem cells. Oxygen reduction to 3-
6% promotes the survival of both peripheral and central
nervous system stem cells, and can influence their fate by
enhancing catecholaminergic differentiation. Similarly,
human hematopoietic stem cells demonstrate increased
self-renewal and bone marrow repopulating capability
after hypoxic treatment. Furthermore, because cartilage
is a relatively avascular tissue, chondrocytes are bathed in
a naturally hypoxic milieu and rely on hypoxia-induced
signaling for survival. Murine marrow-derived mesen-
chymal stem cells (MSCs) undergo enhanced osteo-
chondrogenic differentiation in the setting of chronic
hypoxia, perhaps by returning them to a more ''natural''
oxygen environment. In contrast, preadipocytes do not
thrive under hypoxia, and the absence of oxygen triggers
a hypoxia inducible factor (HIF)-l a response that re-
presses genes essential to adipogenic differentiation.
Thus, although reduced-oxygen incubation may promote
the
in vitro
expansion and/or differentiation of many cell
types by mimicking
in vivo
oxygen levels, it is possible to
inhibit the differentiation of others by exposing them to
reduced oxygen.
attached but has not spread, (2)
intermediate adhesive-
ness
characterized by cell spreading, but which lacks
stress fibers and focal adhesions, and (3)
late adhesiveness
indicating cell spreading with stress fibers and focal ad-
hesions. Early adhesiveness is important since it is the
first step of attachment to substitutes, which results in
cell growth, differentiation, viability, and spreading.
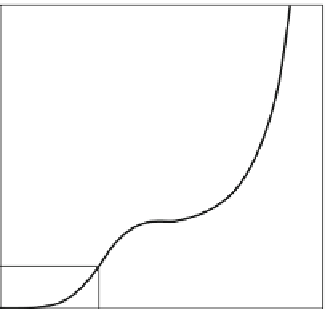
Figure 7.2-19
diagrams the general form and character-
istics of attachment and proliferation rate assays, iden-
tifying quantitative parameters extracted by statistical
fitting to data. Here, %
I
max
measures the maximum
number of cells that attach to a surface from a cell sus-
pension, expressed as percent of inoculum.
Integrins that mainly mediate the biological cell ad-
hesion are involved in processes such as development,
wound healing, tumor invasion, and inflammation. Many
cells appear to be capable of attaching to implant mate-
rials through integrins, and a considerable number of
proteins contain the requisite RGD and (G)RGD(S)
(Gly-Arg-Asp-Ser) sequences which are recognized by
integrins. Serum contains abundant proteins with un-
known activities in terms of cell adhesion. A high con-
centration of FN in serum can serve as an immediate
attachment protein and may anchor cells to implants.
Cell adhesion to a substrate is dependent on the chemical
properties such as material composition and wettability,
and physical parameters such as porosity and roughness.
With the passage of time the cell can produce the ECM,
and the ECM proteins in turn will attach to the sub-
stitute surface.
Cells can also be cultured successfully on a surface
coated with type I collagen, but there is a concern
200
Proliferation
150
k
100
%/
max
50
Adhesion
t
d
t
1/2
0
0.1
1
10
100
Time (h)
Fig. 7.2-19 Schematic illustrating cell adhesion and proliferation
identifying quantitative parameters extracted from the variation of
percentage of a cell inoculum (%I ) with time. %I
max
is the
maximum percentage of a cell inoculum that adheres to a surface
from a sessile cell suspension and t
1/2
measures half-time to
%I
max
. The proliferation rate (k) and cell-number doubling time (t
d
)
measure viability of attached cells.
7.2.6.2.2 Cell adhesion
Cell adhesion can be divided into three grades of adhe-
siveness: (1)
early adhesiveness,
meaning that a cell is