Biomedical Engineering Reference
In-Depth Information
gels. Methods have been developed that allow for colla-
gen materials to directly crosslink without incorporation
of crosslinking reagents. To date, the recognized mecha-
nisms for strengthening collagen constructs in the presence
of cells are nonenzymatic glycation and enzyme-mediated
crosslinking techniques, thereby enhancing mechanical
strength while remaining benign toward the cells.
A number of studies have shown that the major anti-
genic determinants of collagen are located within the
terminal regions. In still other cases, evidence has been
presented to suggest that central determinants also play
a role in collagen-antibody interactions. Collagens are
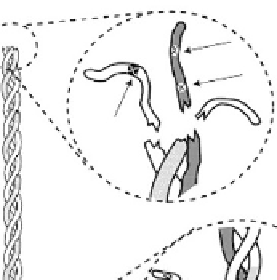
treated with proteolytic enzymes to remove the terminal
telopeptides. However, in some cases, telopeptide rem-
nants persisting following pepsin treatment (
Fig. 7.2-3
)
[1]
have been shown to be sufficiently large so that the
antigenic activity of the pepsin-treated and native forms
is almost indistinguishable. Further detailed study may
be needed to characterize the human immune response
to xenogeneic collagen.
Although several commercial skin products are based
on bovine type I collagen which has been licensed for
clinical use by the Food and Drug Administration (FDA),
this would seem not to be a good long-term solution for
clinical use. The possible risk of virus and prion tends to
reduce the use of collagen in tissue engineering, but the
use of collagen in tissue engineering likely still continues
because of its excellent properties as scaffold. De-
velopment of good assay kits for virus check and rea-
sonable consideration of risk/benefit balance are required
for the safe application of collagen. A big challenge at the
moment is to produce inexpensive human recombinant
collagen that is completely free of any virus and prion.
Besides collagen, elastin plays a major role in de-
termining the mechanical performance of some native
tissues. Elastin fibers can extend 50-70% under physio-
logical loads, and depending on the location of the vessel,
elastin content can range from 33% to 200% that of
collagen. In native tissues, elastin exists in stable fibers
that resist both hydrolysis and enzymatic digestion.
Gelatin
Gelatin is obtained by denaturing collagen by heating
animal tissues including bone, skin, and tendon. Alkaline
pretreatment of collagen, which converts asparagine and
glutamine residues to their respective acids, produces
acidic gelatin with isoelectric points below 7, while ex-
traction with diluted acid or enzymes yields basic gelatin
with isoelectric points higher than 7. Gelatin is a hetero-
geneous mixture of single and multistranded polypeptides,
each with extended left-handed proline helix confor-
mations and containing between 300 and 4000 amino
acids. The triple helix of type I collagen extracted from
skin and bones is composed of two a1 (I) and one a2 (I)
chains, each with MWof w95 kDa, width of 1.4 nm, and
length of 290 nm. Gelatin consists of mixtures of these
strands together with their oligomers and breakdown
(and other) polypeptides. Solutions undergo coil-helix
transition followed by aggregation of the helices by
the formation of collagen-like right-handed triple-helical
C-terminal—full
cleavage of major
antigenic sites
C-terminal
telopeptide
Pepsin
Triple
helical region
treatment
N-terminal
telopeptide
N-terminal—some
major sites remain
uncleaved
Fig. 7.2-3 Telopeptide removal from collagen via pepsin treatment.