Biomedical Engineering Reference
In-Depth Information
is completed. In addition, characterization of the chem-
ical makeup of the degradation products may be possible
through the use of GPC or high-performance liquid
chromatography (HPLC) (
Timmer
et al.,
2002
). How-
ever, such studies do not allow for the continuous obser-
vation of changes within the scaffolds. An
in vivo
study is
often necessary to predict the degradation behavior of the
scaffolds for cell transplantation (Shin
et al.,
2003b).
Material surfaces, which are usually different from the
bulk, play a crucial role in regulating cell response.
Electron spectroscopy for chemical analysis (ESCA) and
static secondary ion mass spectrometry (SIMS) are the
most powerful tools for analyzing surface chemistry and
composition. Information on the orientation of chemical
groups can be obtained by polarized IR and near-edge X-
ray absorption fine structure (NEXAFS). Surface mor-
phology can be characterized by SEM, scanning probe
microscopy (SPM), and atomic force microscopy
(AFM). Surface wettability and energy are assessed by
contact-angle measurements.
A
B
C
Oxygenator
Pump
Medium
Reservoir
Cell/polymer
construct
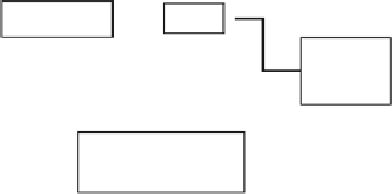
Fig. 7.1.4-4 Dynamic cell seeding and culture techniques in 3D
scaffolds. (A) Spinner flask. (B) Rotary vessel. (C) Perfusion
system.
Cell seeding and culture
in 3D scaffolds
Several dynamic cell seeding and culture techniques
have been developed to ensure uniform cell distribution,
which will lead to uniform tissue regeneration (
Fig.
7.1.4-4
). Compared to static culture conditions, mass
transfer rates can be maintained at higher levels and cell
growth is not restricted by the rate of nutrient supply
under well-mixed culture conditions. These methods can
be scaled up and are suitable for cell cultivation using
multiple scaffolds.
The major obstacles to the
in vitro
development of 3D
cell-polymer constructs for the regeneration of large
organs or defects have been obtaining uniform cell
seeding at high densities and maintaining nutrient
transport to the cells inside the scaffolds. To achieve
desired spatial and temporal distribution of cells and
molecular cues affecting cellular function, cell culture
conditions should provide control over hydrodynamic
and biochemical factors in the cell environment.
Spinner flask culture
In a spinner flask, 3D polymer scaffolds are first fixed to
needles attached to the cap of the flask, and then ex-
posed to a uniform, well-mixed cell suspension
(
Fig. 7.1.4-4
A) (
Freed
et al.
, 1993
). Using this method,
porous PGA scaffolds have been uniformly seeded with
chondrocytes at high yield and high kinetic rate (to
minimize the time that cells stay in the suspension)
(
Vunjak-Novakovic
et al.
, 1998
). Mixing has been found
to promote the formation of cell aggregates with sizes of
20-32
m
m. The spin rate, however, needs to be well
adjusted to minimize cell damage under high shear stress.
The spinner flask is also suitable for suspension culture of
hepatocyte spheroids that exhibit enhanced liver func-
tion compared to monolayer culture in the long-term
(
Kamihira
et al.
, 1997
).
Static culture
The conventional static cell seeding technique involves
the placement of the scaffold in a cell suspension to allow
the absorption of cells. However, the resulting cell dis-
tribution in the scaffold is often not uniform, with the
majority of the cells attached only to the outer surfaces
(
Wald
et al.,
1993
). Wetting hydrophobic polymer scaf-
folds with ethanol and water prior to cell seeding allows
for displacement of air-filled pores with water and thus
facilitates penetration of cell suspension into these pores
(
Mikos
et al.
, 1994a
). Infiltration with hydrophilic
polymers or surface hydrolysis of scaffolds has also been
shown to increase the cell seeding density (
Gao
et al.,
1998; Mooney
et al.,
1995b
). Seeding cells by injection
or applying vacuum to ensure penetration of the cell
suspension through the 3D matrix could result in uni-
form cell seeding initially. However, the uniformity is lost
under static culture conditions because of the nutrient
and oxygen diffusion limitation within the scaffold.
Rotary vessel culture
The rotating-wall vessel (RWV) also allows enhanced
mass
transport
and
is
useful
for
3D
cell
culture